Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Liquid metals catalyze industrial reactions
Gallium-palladium droplets drive alkane dehydrogenation with high selectivity
by Mitch Jacoby
July 28, 2017
| A version of this story appeared in
Volume 95, Issue 31

Gallium’s quirky liquid-state properties have pushed that element into the scientific spotlight recently, as researchers have tapped the liquid metal for applications in stretchable electronics and three-dimensional printing. Now gallium is back in the news, this time as a catalyst.
Researchers in Germany report that liquid droplets of Ga-Pd alloys function as active and durable catalysts for alkane dehydrogenation. That industrial-scale reaction converts low-value alkanes to higher-value olefins, compounds with C=C bonds that are used to make polymers and chemicals (Nat. Chem. 2017, DOI: 10.1038/nchem.2822).
Gallium and some of its alloys exhibit a handful of unique properties, such as a tendency to remain liquid over an enormous temperature range—about 2,000 °C. The metal also has a knack for spontaneously forming an ultrathin oxide skin that stabilizes liquid droplets but easily breaks, allowing the metal to flow momentarily until the skin re-forms around the liquid.
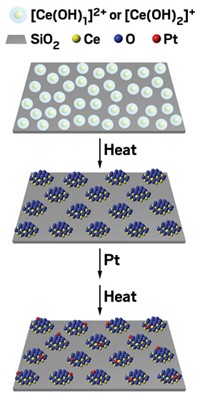
A team including Nicola Taccardi and Peter Wasserscheid of Friedrich-Alexander University, Erlangen-Nürnberg, took advantage of those properties of gallium and its ability to dissolve numerous metals, generating alloys with various concentrations of palladium, a catalytically active metal. Then they deposited the liquid metals onto porous glass, forming supported liquid metal catalysts, and used them in a test reaction: butane dehydrogenation.
Homogeneous, solution-phase catalysts have the advantage of possessing clearly defined active sites and mechanisms. The aim of the new work was to create a hybrid catalyst with these advantages that can also be easily separated from reaction products and reused, a task that’s currently difficult to carry out with homogeneous versions. Various researchers have attempted this feat previously. But the stability of their liquid-phase catalysts typically limited reactions to roughly 200 °C and below, far lower than temperatures required in many industrial catalytic processes.
The Friedrich-Alexander team ran test reactions at roughly 450 °C and found that gallium-rich catalysts, for example, ones with a Ga-to-Pd ratio of 10:1, had high activity for butane dehydrogenation, produced butene with high selectivity (85%), and remained in the liquid state even after 20 hours of reaction. In addition, they did not accumulate the layer of carbon (coke) that gunks up and deactivates commercial Pt-Al2O3 and Cr2O3-Al2O3 dehydrogenation catalysts.
“Supported liquid metal catalysis is an interesting concept,” says Arizona State University’s Jingyue (Jimmy) Liu, a catalysis specialist. He is particularly intrigued by the researchers’ atomic-level description of their catalyst as individual isolated Pd atoms supported on the surface of a Ga-Pd liquid metal. He adds, “Systematic investigations are needed to better understand reaction processes in such a system and to provide deeper insights into the nature of the newly synthesized liquid metal.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter