Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
New borate crystal boosts UV optical applications
Simple synthesis and useful nonlinear optical properties may be a boon for short-wavelength photonics
by Mitch Jacoby
August 20, 2017
| A version of this story appeared in
Volume 95, Issue 33
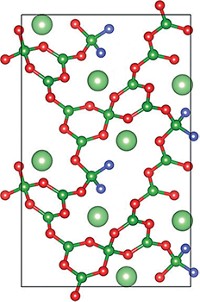
The ability of inorganic crystals known as nonlinear optical (NLO) materials to alter the properties of a beam of laser light—for example, doubling its frequency—makes them indispensable for applications in fiber optics, photolithography, and laser micromachining. Few NLO materials can generate coherent light deep in the ultraviolet range (< 200 nm). KBe2BO3F2 (KBBF) is an exception. But the toxicity of beryllium and the low intensity of KBBF’s NLO properties limit its application. A team of researchers led by Shilie Pan of Xinjiang Technical Institute of Physics & Chemistry and Kenneth R. Poeppelmeier of Northwestern University may have come up with a solution—NH4B4O6F (ABF), a beryllium-free deep-ultraviolet NLO material (J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.7b05943). The researchers report that ABF’s nonlinear coefficients are roughly 2.5 times as large as KBBF’s values. They also note that their synthesis method, based on the high-temperature reaction of B2O3 with NH4F, leads to high-quality crystals that tend to be thicker than typical KBBF crystals, which benefits applications in laser optics. One source of the improved crystal growth is hydrogen bonding between lattice layers, which results from replacing potassium ions in KBBF with ammonium ions in ABF.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter