Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Structure of superlong, ice-binding protein reported
Adhesin protein helps Antarctic bacteria stick to ice and photosynthetic diatoms at same time
by Stu Borman
August 15, 2017
| A version of this story appeared in
Volume 95, Issue 33

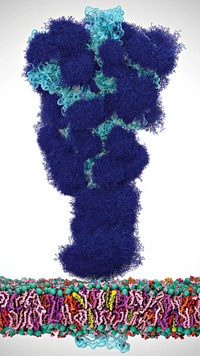
After a decade of persistent effort, researchers have obtained the first complete structure of a bacterial protein with one of the longest folded conformations of any known protein—600 nm in length.
The protein is expressed on the surface of a bacterium called Marinomonas primoryensis, which lives in waters in and around Antarctica. The biomolecule, a so-called adhesin protein named M. primoryensis ice-binding protein (MpIBP), latches onto the underside of ice shelves, a near-surface location where sunlight is plentiful. By attaching to the shelves, the bacteria can cohabit with nearby diatoms, which photosynthesize oxygen the microbes need.
Few proteins are in MpIBP’s size range, one exception being the muscle protein titin, which is more than 1 µm in length. Most folded proteins are 2 to 15 nm long.
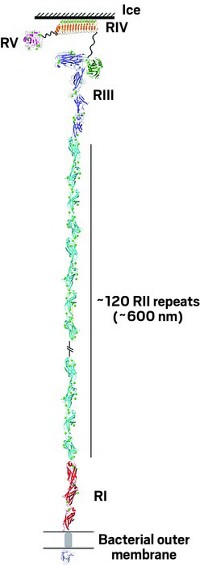
The single protein chain of MpIBP is divided into five regions, called RI, RII, RIII, RIV, and RV. In earlier work, Peter L. Davies of Queen’s University, in Ontario; Ilja K. Voets of Eindhoven University of Technology; and their coworkers determined separate X-ray crystal structures for the RII and RIV regions. Now they have used X-ray crystallography, nuclear magnetic resonance spectroscopy, and small-angle X-ray scattering to structurally analyze the RI, RIII, and RV regions, enabling them to assemble the entire overall structure (Sci. Adv. 2017, DOI: 10.1126/sciadv.1701440).
“This massive protein would have been essentially impossible to study in one piece,” comments Karl E. Klose of the University of Texas, San Antonio. “The dissect-and-conquer approach allowed these researchers to gain insights into how the various modules work together to allow the bacterium to thrive in the Antarctic.”
The structure shows that RI anchors the protein to the bacterium. RII is a long string of about 120 repeating domains that gives the protein most of its extraordinary length. RIII contains domains that enable the protein to hook up with nearby microorganisms such as the bacteria’s diatom neighbors. The protein attaches to ice through RIV. And RV may act as a nucleus to initiate proper folding of the entire protein.
Voets points out that MpIBP grips ice in a way that is similar to how pathogenic bacteria attach to our cells. Understanding the MpIBP attachment process could thus help scientists develop a new way to block human bacterial infections, she says.
Robert Michael L. McKay, director of the Marine Program at Bowling Green State University adds that the study’s detailed characterization of the adhesin’s structure could aid the discovery of new strategies to inhibit biofilm formation, such as bacterial films on medical devices that can cause infections.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter