Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Copper pairs up to reduce nitrogen oxides in diesel exhaust
ACS Meeting News: Unusual catalytic mechanism plays key role in engine emissions cleanup
by Mitch Jacoby
August 23, 2017
| A version of this story appeared in
Volume 95, Issue 34

High schoolers aren’t the only ones who pair up and break up frequently. Catalytic species in the systems that clean up engine exhaust do it too, according to a study presented on Tuesday at the American Chemical Society national meeting in Washington, D.C.
The investigation uncovers an unusual mechanism in the catalytic process that rids diesel engine exhaust of smog-causing nitrogen oxides (NOx). The findings may eventually lead to more effective catalysts.
Diesel-powered engines score high marks for fuel efficiency, which is why nearly all long-haul trucks use them. But they emit various pollutants, including hydrocarbons, CO, and NOx.
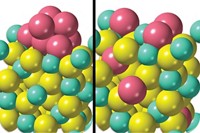
Selective catalytic reduction (SCR) systems—part of the overall exhaust cleanup equipment on diesel vehicles—reduce NOx to nitrogen and water through a reaction with ammonia. The ammonia comes from an aqueous urea solution, which is carried on board like windshield cleaner. SCR systems catalyze NOx reduction with the help of chabazite zeolite that has been treated to incorporate copper in its lattice.
These systems come standard on many diesel vehicles, yet details of how they work remain a subject of debate.
A team led by Rajamani Gounder of Purdue University and William F. Schneider of the University of Notre Dame studied some of the details in copper-chabazite SCRs. Using X-ray spectroscopy, kinetic measurements, and quantum calculations, the researchers tracked unusual behavior of the zeolite’s copper ions.
Speaking at a symposium sponsored by the Division of Catalysis Science & Technology, Gounder reported that in the presence of ammonia, copper ions form Cu(NH3)2 complexes. The NH3 moities make the species mobile, allowing them to migrate through openings that interconnect hollow cages in the porous zeolite framework. As the copper species move about, they briefly and reversibly form dimers that are bridged by a pair of oxygen atoms. The dimers facilitate an O2-mediated Cu(I) to Cu(II) redox step that’s central to reducing NOx to nitrogen and water (Science 2017, DOI: 10.1126/science.aan5630).
Gounder explained that, in effect, individual copper ions come together and work in tandem to carry out the difficult step of breaking apart oxygen molecules. The copper ions then go back to being isolated after the reaction is complete. He noted that this step might be accelerated by fine-tuning the spatial distribution of copper ions in the zeolite, leading to lower NOx emissions at cooler operating temperatures than is possible with current SCR systems.
“Exquisite” is how Robert J. Davis described the team’s techniques for exploring this SCR system. Davis, a catalysis specialist at the University of Virginia, remarked that this exciting finding regarding the mobility of copper ions and the dynamic formation of paired copper species may also be relevant to the high selectivity of Cu-treated zeolites used for oxidizing methane to methanol.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter