Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Petrochemicals
A whole new world for rare earths
How the technologically important metals rose from obscurity to ubiquity
by Stephen K. Ritter
August 28, 2017
| A version of this story appeared in
Volume 95, Issue 34

“Welcome Rare Earthers.”
In 1965, this message appeared on a Holiday Inn sign in Ames, Iowa, to greet the attendees of the 5th Rare Earth Research Conference (RERC). Rare-earth chemistry was at a fulcrum point in its history. Previously, during World War II, chemists working in Ames at Iowa State University had played a critical role in rare-earth technology. As part of the Manhattan Project in 1942, the researchers developed methods to remove traces of rare-earth metals from the uranium used to create the first nuclear chain reaction and to make the first nuclear bombs.

In brief
The rare earths, a collection of 17 elements including the lanthanides—lanthanum to lutetium—along with scandium and yttrium, have become indispensable components in many essential technologies of modern life, including smart phones, LEDs, and medical imaging. But not much was known about rare-earth chemistry until the 1960s. Since then, researchers have uncovered the elements’ abundant magnetic, luminescent, electrochemical, and other properties. The Rare Earth Research Conference, held recently in Ames, Iowa, brought together researchers who discussed the evolution of rare-earth chemistry, new research trends, and the need for sound natural resource management to ensure their sustainable use for future technologies.
Not much was known about the chemistry of the rare earths in the 1940s, or even by the 1960s. This group of 17 elements, which include the lanthanides—lanthanum to lutetium—along with scandium and yttrium, seemed to consist of fairly unreactive metals that all behaved similarly. Most of the research and development involving the elements was metallurgical, not chemical. Until 1965, the major applications for the rare earths included using mixtures of rare-earth oxides for polishing lenses and mirrors, using cerium and lanthanum oxides as promoters in zeolite catalysts for petroleum refining, and using “mischmetal” rare-earth alloys with iron as flints for lighters.
Flash forward to this past June, and rare earthers were back in Ames for the first time since 1965, for the 28th RERC. The Holiday Inn sign is long gone from the still-cozy midwestern college town, and the world of rare earths has changed.
In 1965, the “counterculture revolution” was under way, and many of the world’s young adults were protesting in favor of peace over nuclear power and the nuclear weapons of the Cold War. At the same time, they were falling in love with modern conveniences, driving demand for microwave ovens, early versions of personal computers and video games, and televisions. Coincidentally, 1965 witnessed the launch of the first major commercial use for a highly purified rare-earth material: Europium-doped yttrium salts began to be used as red phosphors in the picture tube of color television sets.
Now 52 years on, the internet rules nearly everyone’s lives, and handheld devices have greater capabilities than those early bulky computers and televisions. Much of the technology evolution since the 5th RERC has come about as researchers discovered that rare earths have abundant magnetic, luminescent, electrochemical, and thermal properties that have made possible smart phones, electric cars, light-emitting diodes, wind turbines, medical imaging, and more. This year’s RERC served as a grand review of this rare-earth chemistry, highlighting how adding a dash of rare-earth metals to materials is like adding a bit of magic fairy dust—the metals help everything perform better.
“The remarkable properties of the rare earths have long engaged the imagination of the scientific community,” said rare earther Ana de Bettencourt-Dias of the University of Nevada, Reno, one of this year’s conference organizers. She marveled at the metals’ importance in applications as varied as automobile starter motors, audio speakers, lasers, and colored glass.
The conference revealed new research trends showing that rare-earth chemistry is still very much a scientific frontier of the periodic table, with further technological developments to come. The discussion in Ames also turned to the need for better environmental stewardship of rare-earth element resources to ensure their sustainable, socially responsible use in existing and future technologies.
Although labeled as a “rare earth” conference, scientists in Ames included the actinides such as uranium on the agenda, given the kinship between lanthanides and actinides as fellow f-block elements. The actinides are increasingly being used in nuclear medicine and as catalysts.

The f-element relationship is actually how rare-earth chemistry got started. For the Manhattan Project, scientists needed pure uranium, lots of it, fast and at a low price. Rare-earth impurities in uranium could prevent a nuclear reactor from working by absorbing the neutrons needed to keep the chain reaction going. So the rare earths had to be removed. Yet the chemical similarity of the lanthanides makes it extremely difficult to isolate them from one another and to separate them from uranium and other actinides. Iowa State chemistry professor Frank H. Spedding, an expert on rare earths, helped guide solution processing efforts to make tons of pure uranium oxide and a new high-temperature smelting method—called the Ames process—to make tons of pure uranium metal.
After WWII, in 1947, Spedding helped create and became director of Ames Laboratory, a U.S. Department of Energy national laboratory with a mission to further develop rare earths, initially for military uses and space exploration. Building on their earlier work, Spedding and his team developed an ion-exchange method to extract rare earths from minerals, enabling better separation of the rare-earth metals on a larger scale.
“Rare-earth research became commonplace after the groundbreaking work performed in Ames in the 1940s and 1950s,” explained materials scientist Vitalij Pecharsky of Iowa State and Ames Laboratory, another RERC organizer. “Spedding and his colleagues made pure rare earths available in quantities required for broad chemistry and physics research and applications for the first time.”
Pecharsky said his own research developing new rare-earth magnetic materials was made possible by those pioneering efforts. For example, his work with one of those pioneers, Karl A. Gschneidner Jr., who passed away last year and was memorialized at the conference, led to the invention of Gd5Si2Ge2. This material’s temperature, Pecharsky explained, can be altered by exposing it to a changing magnetic field. Such magnetocaloric materials are being developed for high-efficiency refrigeration that uses a quarter of the energy of current systems.
“Rare earths remain a favorite playground for both basic and applied science,” Pecharsky said, “driven by the potential for their everyday applications.”
Kenneth N. Raymond of the University of California, Berkeley, another rare-earth-playground leader, was recognized during the conference as this year’s Spedding Award winner, a prize that honors outstanding achievement in rare-earth research and education. Raymond’s award lecture, “Lanthanides in Bondage: From Basics to Business,” which led off the conference, reviewed his 40-plus-year career studying lanthanide luminescence and developing biomedical applications for the rare earths.
Starting out, Raymond worked with other leading researchers of the day—UC Berkeley’s Glenn T. Seaborg and Andrew Streitwieser—to determine crystal structures of new lanthanide and actinide complexes. “Although there were a number of these compounds known, there was almost no structural information about them,” Raymond said. “So I set about to change that.” Raymond developed predictive models for the structure and bonding of lanthanide and actinide compounds based on the ionic radii of the metals.

Subsequent studies have enabled chemists to better understand the electronic transitions possible in lanthanide complexes when they are excited by light, and helped researchers design sequestering agents for plutonium and other heavy elements used to prepare and reprocess nuclear fuels.
Although lanthanides are weaker light emitters than organic dyes such as rhodamine B and fluorescein, Raymond said, they provide a technical advantage because their luminescence lasts longer, which allows better detection. Understanding the photochemistry of these lanthanide complexes when they are attached to drugs, proteins, and antibodies has proved valuable for developing new types of diagnostic assays and medical imaging.
In 2001, Raymond cofounded Lumiphore to commercialize terbium, europium, and other lanthanide luminescent complexes. These complexes are now used in assay kits sold by other companies to test saliva, blood, or tissues for drugs of abuse, hospital bacterial infections, and fetal and neonatal diseases. The technology is also being developed for biological imaging—for example, to study protein-protein interactions in living cells.
“We have learned a lot about the fundamental coordination chemistry of lanthanides over the years, and it’s still an interesting subject for research,” Raymond said. “Maybe the biggest thing for me is that if you put these metals in bondage, that is, incorporate them inside chelating ligands, the chemistry can be used to practical effect.”
William J. Evans, another rare earther, agrees. Evans and his coworkers at the University of California, Irvine, are one of several research groups who have been designing new metal complexes that are shaking up the conventional wisdom about rare-earth element oxidation states. Their findings point to new approaches for manipulating the magnetic, optical, and catalytic properties of rare earths.
“When I started working with rare-earth metals in the 1970s, they were considered an exotic part of the periodic table—people had to stop and think about where those metals are positioned,” Evans told C&EN. “My research advisers thought I was crazy to go to work in this area, because there was no demonstrated importance in the chemistry of these elements.” But Evans said the dearth of work on lanthanides was their allure.
Rare earths at a glance
| NAME | SYMBOL | ATOMIC NUMBER | ELECTRON CONFIGURATIONa | ETYMOLOGY | NOTABLE APPLICATIONS |
|---|---|---|---|---|---|
| Scandium | Sc | 21 | 3d14s2 | Scandia (Latin for Scandinavia) | Aluminum alloys, lighting |
| Yttrium | Y | 39 | 4d15s2 | Ytterby, Swedenb | Yttrium-aluminium-garnet lasers, red phosphors, cancer drugs |
| Lanthanum | La | 57 | 4f05d16s2 | Lanthanein (Greek, meaning to lie hidden) | Refinery catalyst, camera lenses, lighter flints |
| Cerium | Ce | 58 | 4f15d16s2 | Ceres, the dwarf planet, and Roman goddess of agriculture | Car catalytic converters, glass polishing agent, lighter flints |
| Praseodymium | Pr | 59 | 4f35d06s2 | Prasios and didymos (Greek, meaning leek-green twin) | Magnets, lighter flints, greenish-yellow glass and ceramics |
| Neodymium | Nd | 60 | 4f45d06s2 | Neo and didymos (Greek, meaning new twin) | Nd2Fe14B magnets, lasers, violet glass and ceramics |
| Promethium | Pm | 61 | 4f55d06s2 | Greek Titan Prometheus | Radioactive; luminous paint, pacemaker batteries |
| Samarium | Sm | 62 | 4f65d06s2 | Vasili Samarsky-Bykhovetsc | SmCo5 magnets, cancer therapy, nuclear reactor control rods |
| Europium | Eu | 63 | 4f75d06s2 | Europe | Red phosphors for lighting and color displays |
| Gadolinium | Gd | 64 | 4f75d16s2 | Johan Gadolind | Refractive glass, MRI contrast agent, nuclear reactor shielding |
| Terbium | Tb | 65 | 4f95d06s2 | Ytterby, Swedenb | Green phosphors for lighting, magnetorestrictive alloys |
| Dysprosium | Dy | 66 | 4f105d06s2 | Dysprositos (Greek for hard to get) | Stabilizing additive in magnets, lasers |
| Holmium | Ho | 67 | 4f115d06s2 | Holmia (Latin for Stockholm) | Lasers, magnets |
| Erbium | Er | 68 | 4f125d06s2 | Ytterby, Swedenb | Lasers, fiber optics, nuclear reactor control rods |
| Thulium | Tm | 69 | 4f135d06s2 | Mythological land of Thule | Portable X-ray source, light filaments, lasers |
| Ytterbium | Yb | 70 | 4f145d06s2 | Ytterby, Swedenb | Lasers, chemical reducing agent, stainless steel additive, cancer therapy |
| Lutetium | Lu | 71 | 4f145d16s2 | Lutetia (Latin for the city that became Paris) | PET scan detectors, refractive glass, refinery catalyst |
a Ground shells, NIST data. b One of four new elements in the first discovered rare-earth ore found near Ytterby, Sweden, credited to Carl Arrhenius. c Mining engineer who discovered samar- skite, a mineral containing samarium, the first element named after a person. d Gadolinium is named in honor of this rare earther who first identified yttrium in a sample sent from Carl Arrhenius.
Today, the attitude toward rare earths is much different, Evans said, because people have heard of their many uses. “So now when I say I am a rare-earth chemist, people have an idea that these are useful, interesting, and strategically important metals.”
Conventional wisdom among chemists until recently held that most rare earths exist only in the +3 oxidation state in molecular complexes. Each of the 17 elements in the rare-earth collection have three ionizable electrons—two s electrons and one d electron. Moving across the row of lanthanides from lanthanum to lutetium, each element has an additional electron that takes up a position in a 4f orbital. Because the 4f orbitals don’t extend far enough from the atom, they tend not to directly participate in bonding, yet they still influence the elements’ magnetic and optical properties. That’s a broad, simplistic view; the real situation “is complicated,” Evans said.
Researchers found early on with rare earths that cerium readily assumes a +4 oxidation state in compounds, and later researchers began to find that other elements could take on +2 or possibly +5 oxidation states. By the late 1990s, chemists had isolated lanthanide(II) complexes for six of the elements. At the time, the researchers rationalized their results by noting the ions were stabilized by having filled, nearly filled, half-filled, or nearly half-filled 4f orbitals.
But as researchers continued to investigate, they discovered that ligands with the right electronic properties could provide additional stability, leading to additional +2 complexes. In 2013, Evans and Matthew R. MacDonald in his group reported complexes for the final seven elements, completing the lanthanide(II) series to show that it could be done. The team actually didn’t make the promethium complex, because the element is radioactive and doesn’t occur naturally. But the researchers expect it should follow suit and form a +2 complex.
The lanthanide(II) complexes are especially interesting, Evans noted, because they are kind of a hybrid between transition metals and lanthanide metals. They don’t appear to have a 6s electron, but rather a 5d electron like a transition metal, in addition to any 4f electrons. And they display one-electron redox chemistry, which is not always typical for transition metals. “It’s like they are a new type of metal,” he said.
Evans and his colleagues have since found that the dysprosium(II) and holmium(II) complexes exhibit the highest magnetic moments reported to date for any single metal ion complex. They haven’t figured out how to use this breakthrough practically yet, Evans said. One idea is that these complexes could function as single-molecule magnets and be used in thin-film coatings for computer hard drives to increase the density of data storage.
As for what’s next with rare-earth chemistry, Evans asked: Why not try to make +1 rare-earth complexes? He thinks as chemists continue to tinker with new electron configurations, they should be able to further elaborate on rare-earth technologies. “Hopefully, future research will allow us to use these metals more efficiently and reduce the tremendous demand on them for their special properties.”
Advertisement
As Evans alluded, the demand for rare earths could lead to their commercial demise. Access to the metals has always been tenuous, leading to worries that one day demand for them may outstrip supply.
To head off that scenario, Gisele Azimi of the University of Toronto, another rare earther speaking at the conference, sees an opportunity for developing better strategies to ensure sustainable supplies of rare earths. These include finding new sources of the metals, developing better extraction technology for them, improving the efficiency of rare-earth-metal usage in products, and closing the loop on their use with better recycling.

Despite their name, rare-earth elements are not rare. All the metals except radioactive promethium are actually more abundant in Earth’s crust than silver, gold, and platinum. When the first rare earths were discovered in the late 18th century, they were found as complex mixtures of metal oxides in various minerals. The minerals were initially, and incorrectly, classified as “earths,” a geological term denoting nonmetallic substances that are insoluble in water and resistant to chemical change by heating. Furthermore, because of their geochemical properties, rare-earth elements don’t concentrate in ore deposits but are widely dispersed, so they only seemed to be scarce.
Global rare-earth reserves, at more than 130 million metric tons, appear to be ample, Azimi noted. However, most of those reserves either are too low in concentration to be extracted economically, or they are not readily accessible, such as metals locked away in deep-sea manganese-based nodules or hydrothermal deposits.
“Currently, the demand for rare earths is increasing at a rate of about 5% annually,” Azimi said. Some rare-earth applications require large amounts of the metals: Wind turbines use some 600 kg of rare-earth metals apiece. Some uses need intermediate amounts: Electric vehicle batteries use 5 to 10 kg of rare earths each. And some uses require small amounts: Phosphors in lighting may use a fraction of a gram per bulb.
The key issue at the moment is that China is home to about 25% of the world’s rare-earth reserves, yet economic forces have aligned so that China now accounts for roughly 90% of the global supply. This situation has made sourcing rare earths precarious, subject to price and supply swings that are disruptive to product manufacturers.
That leaves mining companies outside China caught in an industrial catch-22: If they want to grab a larger piece of the market, the companies must ramp up production of rare earths, but they must do so without saturating the market with supply to avoid lowering prices. While companies deal with those economic conditions, they are also increasingly being judged for their environmental stewardship and their social responsibility to the communities they operate in.
The U.S., Australia, Brazil, and Canada have large reserves of rare earths, Azimi said, but little active mining is currently going on in those countries because of the cost. The real challenge going forward, she said, is going to be scaling up supply at a rate that matches increases in demand. “Opening new mines takes time—a decade or more—for prospecting, permitting, and construction. It’s expensive and has environmental downsides.” From digging in the ground to producing pure rare-earth oxides and pure metals, the process uses a lot of water, acids, organic solvents, and extraction chemicals.
One solution Azimi is promoting is to rescue rare-earth metals from existing stockpiles of mining wastes. For example, she is working with residue called red mud from bauxite mining to produce aluminum, and leftovers called phosphogypsum from phosphate rock mining that makes fertilizer. These materials contain 300 to 500 ppm of rare-earth metals, which is more concentrated than the roughly 0.2 to 60 ppm levels of the elements found in natural mineral deposits. With millions of tons of these waste materials piled up globally, thousands of tons of rare-earth metals could be procured, she said. Other researchers at the conference discussed coal ash and spent nuclear fuel from power plants as additional secondary sources of rare earths.
Rare earths are also plentiful in electronic waste, Azimi explained. Used fluorescent lights, computer hard drives, large permanent magnets, and batteries tend to end up in landfills. “Only 1% of their rare-earth content is being recycled,” Azimi said. “It is absolutely imperative to develop recycling processes to address the sustainability challenges associated with these critical materials.”
That’s a big challenge for e-waste because most consumer products aren’t designed to be recycled. In addition, the metals are often used in combination for specific applications and need to be separated again. For example, neodymium-based permanent magnets (Nd2Fe14B) contain some dysprosium to improve thermal performance.
Azimi’s group is working toward a designer acid-leaching process to recover rare earths from red mud and phosphogypsum. At RERC, she described how her team is further using the metals to design rare-earth-containing materials for new commercial uses. For example, the researchers are working on rare-earth oxide ceramics as transparent waterproof coatings for applications such as heat exchangers, airplanes, and solar panels. These materials use a minimal amount of rare earths and they are thermally stable, so they last longer than other types of hydrophobic coatings, which could help address the rare-earth supply challenge, she said
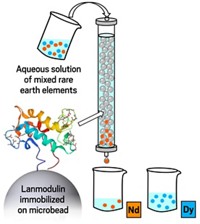
Other methods being used or tested to recycle e-waste include traditional coordination and chelation chemistry, ionic liquid extraction, metal-adsorbing bacteria, and the Ames high-temperature metallurgical process. For example, Eric J. Schelter of the University of Pennsylvania and his group have been developing a new type of targeted metal separation process that uses tailored nitroxide ligands to efficiently separate mixtures of rare-earth metals when recycling magnets and lighting phosphors.
“Rare earths have become indispensable and, in many cases, irreplaceable components of materials that are essential in modern life,” said Schelter, one of the conference organizers. “Moving forward, I’m excited to see the magnetic properties of rare-earth molecules and molecular clusters reach their potential, for f-element complexes to catalytically drive multielectron transformations, and for the realization of highly efficient chemistries for recycling rare earths from consumer materials.”
Technical solutions to the long-term sustainable use of rare earths undoubtedly will come from further research and design of new materials, such as core-shell nanoparticles that use just a dash of rare earths or even thin films of single molecules that achieve the same magnetic, optical, and other magical rare-earth properties as bulkier materials. Some researchers are looking to rare earths as a guide to exploit transition- and main-group metals to mimic rare-earth properties. Those developments might be on the agenda the next time rare earthers reunite in Ames.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter