Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
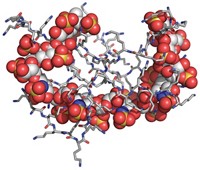
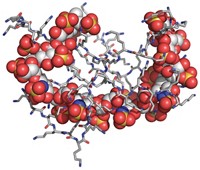
New synthetic heparin could improve blood-thinning treatments
Optimized sulfated polysaccharide could be safer or have more-reversible activity than existing synthetic and animal-derived versions
by Stu Borman
September 11, 2017
| A version of this story appeared in
Volume 95, Issue 36

A new synthetic version of the sulfated polysaccharide heparin that could be safer or have more-reversible activity than currently approved versions of the anticoagulant has been produced in quantities that begin to approach those needed for commercialization. Three types of heparins are currently approved as blood thinners to treat clotting disorders and prevent clotting in people undergoing kidney dialysis and surgery. Unfractionated heparin and low-molecular-weight heparin, derived from pig intestines, are mixtures with batch-to-batch inconsistencies, and contaminated batches have at times killed people. A synthetic heparin called fondaparinux is safer and homogeneous. But people taking heparin sometimes experience uncontrolled bleeding, and although a drug called protamine can reverse the anticoagulant activity of unfractionated heparin, it does so incompletely with low-molecular-weight heparin and doesn’t reverse fondaparinux activity at all. Robert J. Linhardt of Rensselaer Polytechnic Institute, Jian Liu of the University of North Carolina, Chapel Hill, and coworkers recently used chemoenzymatic synthesis to make a synthetic heparin that is less prone to contamination, more consistent, and more amenable to protamine reversal than other heparins are. But they could produce only milligram amounts. Now, a group led by Linhardt, Liu, and UNC’s Rafal Pawlinski has added a sulfate that makes the heparin easier to synthesize, boosted the activity of two key enzymes, and found a way to produce cofactors in higher yield. The redesigned heparin, called 12-mer-1, can be produced in gram amounts and retains protamine reversibility. The researchers tested it successfully in mice and monkeys (Sci. Transl. Med. 2017, DOI: 10.1126/scitranslmed.aan5954).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter