Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
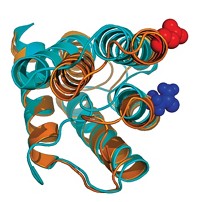
Revised view of Huntington’s protein misfolding mechanism
Study of monomeric protein seems to invalidate ‘rusty hinge’ mechanism
by Stu Borman
October 2, 2017
| A version of this story appeared in
Volume 95, Issue 39
Huntington’s disease, a lethal neurodegenerative condition, is believed to be caused by misfolding of mutated versions of huntingtin protein in which a glutamine-containing sequence is repeated too many times. But how the protein misfolds is still uncertain. Researchers have speculated that the conformation of monomeric huntingtin undergoes a sharp transition when the number of glutamine repeats exceeds 36 or 37, making the domain inflexible, like a rusty hinge. However, mutant huntingtin monomers have been difficult to study because they aggregate rapidly. Hilal A. Lashuel of the Swiss Federal Institute of Technology, Lausanne (EPFL), Rohit V. Pappu of Washington University in St. Louis, Edward A. Lemke of the European Molecular Biology Laboratory, and coworkers have now used protein semisynthesis, single-molecule fluorescence resonance energy transfer spectroscopy, and atomistic computer simulations to structurally characterize huntingtin monomers (J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.7b06659). The study reveals that the monomers have a tadpolelike structure with a globular head and flexible tail. Glutamine repeats are in the head, which enlarges gradually as the repeat number increases instead of being part of a sharp structural transition. The researchers propose that this enlargement, rather than a rusty hinge, may cause misfolding.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter