Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Bayer, Loxo to jointly develop TRK inhibitors
Cancer drug pact could be worth up to $1.5 billion
by Lisa M. Jarvis
November 14, 2017
| A version of this story appeared in
Volume 95, Issue 46
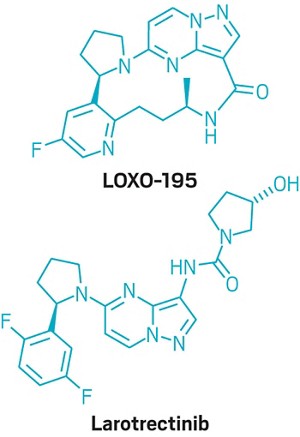
In a deal worth up to $1.5 billion, Bayer and Loxo Oncology will jointly develop two Loxo drug candidates, larotrectinib and LOXO-195, for treating cancers caused by a rare genetic mutation. Loxo gets $400 million upfront; the remainder of the money is tied to the approval and sale of the two compounds.
Larotrectinib and the follow-on compound LOXO-195 both block TRK gene fusions, a chromosomal mismatch found in less than 1% of cancers. Larotrectinib was a breakout star of the American Society for Clinical Oncology conference in June, where researchers revealed that it shrank tumors in 76% of the kids and adults in a small study. LOXO-195, currently in Phase I/II trials, is designed to treat people who develop resistance to first-generation TRK inhibitors like larotrectinib.
Loxo plans to file for U.S. Food & Drug Administration approval of larotrectinib by the end of the year. Whereas oncology drugs have historically been approved based on where tumors are located—such as the lung or breast—Loxo is asking FDA to allow its molecule to be used by anyone harboring TRK gene fusions. Earlier this year the agency granted its first such “tissue agnostic” green light, for an immuno-oncology drug from Merck & Co.
Although the deal is lucrative for Loxo, news of it pushed the company’s share price down by more than 11% today. The biotech firm previously said it would market its TRK inhibitors itself and earlier this month reiterated its progress in setting up an internal team to prepare for the launch of larotrectinib.
Now, Bayer, which some investors view as a second-tier player in oncology, will split U.S. marketing responsibilities with Loxo.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter