Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biocatalysis
Chemists conquer oxidative dearomatization with trio of enzymes
Team leveraged each enzyme’s reactivity to expand the reaction’s scope
by Tien Nguyen
November 17, 2017
| A version of this story appeared in
Volume 95, Issue 46
Enzymes often elicit envy from organic chemists. Highly evolved by nature, these biocatalysts can carry out reactions with a selectivity that surpasses most synthetic catalysts. Yet they act on such a narrow range of substrates that chemists haven’t been able to exploit their abilities in a general way.
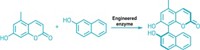
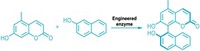
In an effort to broaden enzymes’ reach, researchers led by Alison Narayan at the University of Michigan explored how three known enzymes—called TropB, AzaH, and SorbC—carry out the same reaction (Nat. Chem. 2017, DOI: 10.1038/nchem.2879).

In the reaction, known as an oxidative dearomatization, a catalyst breaks the aromaticity of a planar ring system to install an oxygen species, forming a valuable chiral building block. Narayan and her team determined which of the three enzymes worked best with which substrates from a group, allowing them to maximize the reaction’s enantioselectivity and site selectivity and enabling the chemists to target specific positions and geometries around the ring.
Previous catalytic synthetic methods for oxidative dearomatizations have struggled to achieve high selectivity, often wasting chiral oxidants like hypervalent iodine reagents and metal complexes in the process. The enzyme-driven reaction, on the other hand, uses a more atom-economical reagent, molecular oxygen. Under the conditions needed for synthetic oxidative dearomatizations, products can undergo undesirable side reactions, whereas under the enzymes’ biological reaction conditions, products are stable, Narayan says.
By using freeze-dried cells, which lasted in a freezer for six months without the enzymes inside losing reactivity, the researchers could also run the enzyme reactions at a scale that’s practical for synthetic chemists.
“This is a great example of how enzymes can effect difficult transformations in chemical synthesis,” says Frances H. Arnold, a biosynthesis pioneer at California Institute of Technology. “Starting from newly-discovered enzyme activities, Narayan and coworkers leveraged nature’s diversity to assemble and demonstrate a versatile platform for site- and stereoselective oxidative dearomatization, all the way to the gram scale.”
The team plans to further probe the enzymes’ chemistry with an eye toward eventually engineering the biocatalysts for a broader range of reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter