Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Celanese will acquire the nylon compounding business of the Israeli firm Nilit for an undisclosed sum. Celanese says the purchase will extend its role as a supplier of engineered materials.
Chemtura’s Great Lakes Solutions business has opened a 1,000-m2 pilot plant at its bromine products facility in El Dorado, Ark. The company says the new plant will create six high-paying R&D jobs.
DIC, the Japanese dyes and pigments producer, will pay $229 million for a 19.5% stake in Taiyo Holdings, a maker of materials used in printed circuit boards. Taiyo employs 1,200 people and recorded sales of $440 million in its latest fiscal year.
Janssen R&D has formed two microbiome science collaborations. With DayTwo and Israel’s Weizmann Institute of Science, Janssen will pursue microbiome-based treatments for metabolic disorders. And it is backing Caelus Health, a Belgian firm developing treatments for obesity-associated type 2 diabetes.
Astellas Pharma has licensed Auration Biotech’s AU-935, a treatment for eardrum perforation that is based on heparin-binding epidermal growth factor-like growth factor. The drug is being developed as a topical application to replace surgery.
Cytokinetics has sold some of the potential royalties on omecamtiv mecarbil, an investigational heart drug, to Royalty Pharma for $90 million. Cytokinetics put $40 million of the cash into Phase III development of the drug with its partner Amgen in exchange for higher royalties from Amgen.
Spero Therapeutics has acquired a set of antibacterial candidates from Pro Bono Bio’s Cantab Anti Infectives subsidiary. They join two compounds Spero is developing to treat Gram-negative bacterial infections.
Takeda Pharmaceutical will pay Exelixis $50 million for rights to develop cabozantinib, Exelixis’s leading oncology medicine, in Japan. Cabozantinib was approved in the U.S. last year for the treatment of advanced renal cell carcinoma.

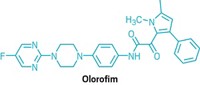
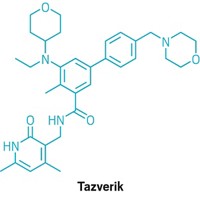
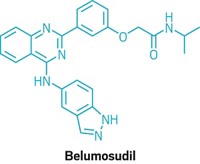

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter