Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New mechanism for a methylating enzyme
Radical SAM enzyme uses unexpected molecule as a methyl donor
by Celia Henry Arnaud
February 3, 2017
| A version of this story appeared in
Volume 95, Issue 6
A team of researchers in China has found a reaction mechanism unprecedented for the many enzymes that add methyl groups to biomolecules.
Most of these so-called methyltransferases depend on S-adenosylmethionine (SAM) as a methyl donor, plucking off the molecule’s methyl group and relocating it either through an SN2 mechanism or through a radical mechanism. Many scientists thought this type of SAM-dependent enzyme exclusively produced S-adenosylhomocysteine as a coproduct during the transfer.

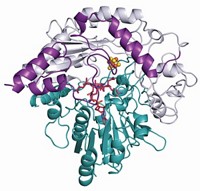
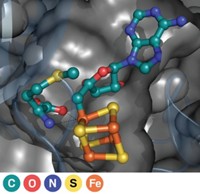
A team led by Qi Zhang of Fudan University reports that’s not always the case. NosN, a class C radical SAM methyltransferase involved in the biosynthesis of a thiopeptide antibiotic, uses a different methyl donor and produces thioadenosine as a coproduct (Angew. Chem. Int. Ed. 2017, DOI: 10.1002/anie.201609948). Knowledge of this mechanism could help researchers reengineer the enzyme to produce other compounds, Zhang says.
Although NosN uses two SAM molecules, neither of them serves directly as the methyl donor. Instead, one SAM molecule is converted into a 5′-deoxyadenosine (dAdo) radical, which initiates the reaction. The other SAM molecule is converted into 5′-methylthioadenosine (MTA). It is this MTA that acts as the methyl donor. Thioadenosine is released as a coproduct.
Previously, researchers thought the dAdo radical abstracted a hydrogen atom from SAM to produce a radical cation, but that’s an energetically unfavorable process.
“Our study shows that nature has solved this problem by simply converting SAM to MTA as a direct donor,” Zhang says.
The finding is “potentially exciting,” says Squire J. Booker, a chemistry professor at Penn State University whose group elucidated the mechanism for class A radical SAM methylases. “No one really liked the idea of a 5′-deoxyadenosyl radical abstracting a hydrogen atom from another molecule of bound SAM” because of the large amount of energy needed to break the bond, he says.

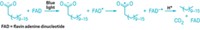

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter