Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biologics
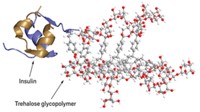
Polymer improves stability of protein drug
A trehalose-based glycopolymer protects insulin against heat and mechanical stress, prolonging the protein’s in vivo lifetime in mice
by Melissa Pandika
February 3, 2017

Attaching biocompatible polymers like polyethylene glycol (PEG) to therapeutic proteins can prolong their lifetime in the bloodstream. But many of these polymers don’t stabilize protein drugs outside the body. Protein drugs often require refrigeration and careful handling during storage and transport; otherwise, they can become inactive, with life-threatening consequences for patients.
Now, researchers have developed a polymer that could stabilize protein drugs both before and after injection. When attached to insulin, the glycopolymer, whose sidechains contain the disaccharide trehalose, prolonged the protein’s lifetime in mice without toxic effects. It also prevented insulin from aggregating in a test tube when exposed to heat and mechanical stress, conditions that can occur during handling and shipping (Bioconjugate Chem. 2017, DOI: 10.1021/acs.bioconjchem.6b00659).
Heather D. Maynard of the University of California, Los Angeles, and colleagues had already found that the trehalose glycopolymer could protect lysozyme and other enzymes from heat and freeze-drying. In the new study, they wanted to test the glycopolymer’s ability to stabilize protein drugs in and out of the body. They focused on insulin, widely used to regulate blood glucose levels in people with diabetes.
After finding that the glycopolymer protected insulin from heat, mechanical stress, and aggregation, even when the two components were simply combined in a tube, the team tested insulin-glycopolymer conjugates, attaching two glycopolymer chains to the protein at different sites. The conjugate turned out to be just as stable as the insulin-glycopolymer mixture when exposed to the same heat and agitation tests.
Injected into mice, the insulin-glycopolymer conjugate had about the same lifetime as an insulin-PEG conjugate—a combination known to increase the lifetime of insulin in vivo. The insulin-glycopolymer conjugate lowered blood glucose levels in mice but required a fivefold higher dose than insulin alone. Earlier studies had reported similar results with insulin-PEG, possibly because the polymer blocks insulin from binding to its receptor. Future research could identify glycopolymers to conjugate to the insulin protein that better enable receptor binding, according to the researchers.
“This is a promising alternative to PEG,” the staple polymer conjugated to protein drugs, says Theresa M. Reineke of the University of Minnesota. Maynard has already begun testing the trehalose glycopolymer with other therapeutic proteins. Stabilizing these drugs is important in the U.S., she says, but also in other areas where continuous refrigeration isn’t available. Still, longer-term preclinical studies are needed. “In-depth mechanistic studies are probably warranted in a future study, as well, to fully understand this system,” Reineke says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter