Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Gene Therapy
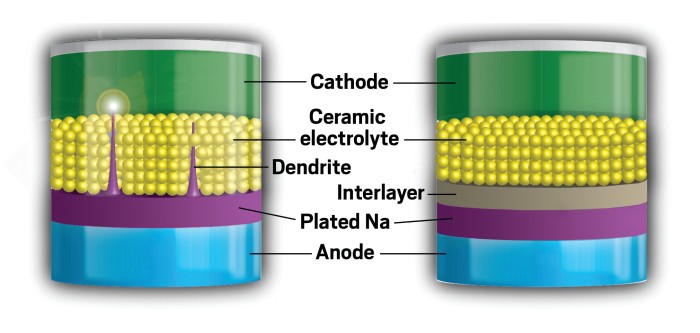
A solid new approach to sodium batteries
Adding an extra layer to solid-state sodium-ion batteries could make them a safe bet for renewable energy storage
by Prachi Patel
February 8, 2017
Lithium-ion batteries are the standard power source for electronics. But lithium is neither cheap nor plentiful, making Li-ion batteries impractical for larger applications, such as storing wind and solar power on a large scale. Sodium-ion batteries are a promising low-cost alternative for such storage, but the ones made so far suffer from low energy density, short battery life, and safety concerns. In an important step toward overcoming these issues, scientists have made a rechargeable solid-state sodium battery that has good efficiency and cycle life, and prevents dangerous overheating from occurring (ACS Cent. Sci. 2017, DOI: 10.1021/acscentsci.6b00321).
Na-ion batteries work like their lithium cousins. During charging, metal ions flow from the cathode, typically a sodium-containing compound, to the anode, typically carbon, through the electrolyte, an organic solvent with dissolved sodium salts. The flow reverses during discharge.
Li-ion battery pioneer John B. Goodenough of the University of Texas, Austin, has been pursuing sodium batteries with a metal anode, instead of carbon, and a solid electrolyte. Sodium metal anodes can store four times as much charge as carbon ones, which means the battery can hold and release more energy. Solid electrolytes, meanwhile, avoid the explosion risks from volatile, flammable solvents, and make batteries safer at high temperatures.
Goodenough, Yutao Li, and their colleagues made a test battery using a thin piece of sodium foil as the anode and a piece of aluminum foil coated with sodium titanate phosphate as the cathode. They made a pellet by fusing together nanospheres of the ceramic Na3Zr2(PO4)(SiO4)2 to serve as the solid electrolyte. This ceramic boasts high sodium-ion conductivity. But when the researchers used it previously in batteries, tiny dendrites grew from the sodium anode and penetrated the electrolyte to reach the cathode, causing an electrical short that overheated the battery.
Dendrites crop up because of an uneven flow of ions across the interface between the anode and the electrolyte, explains Weidong Zhou, who led the project as a senior researcher in Goodenough’s lab. That uneven flow occurs because it’s hard to get close contact between the solid sodium anode and solid electrolyte at the nanometer scale.
So the team came up with two simple tricks to stifle unwanted dendrites by improving the contact between the electrolyte and the electrodes. One was to sandwich the ceramic electrolyte pellet between two ultrathin poly(ethylene glycol) methyl ether acrylate layers. An alternative approach was to melt sodium metal onto the pellet at 380 °C for half an hour. The molten sodium increases the contact between the solid anode and the ceramic pellet.
The cells operated smoothly, showing a capacity of 110 mAh/g and retaining 99% of this capacity for more than 70 charge-discharge cycles. This is better than other solid sodium batteries made so far, but the device is still far from practical. A major issue is its low energy density of 100 Wh/kg—lithium batteries have energy densities over 180 Wh/kg—and low operating voltage. Zhou says that the team is now trying to develop a high-voltage polymer electrolyte that improves cycle life and energy density of the batteries.
This is a significant advance in solid-state Na-ion batteries, says Linda F. Nazar, a chemistry professor at the University of Waterloo. Sodium is more prone to dendrite growth than lithium, and the new proof-of-principle cell takes a big step toward solving that problem. “I hope to see this approach be successful at higher currents, where dendrite formation is exacerbated,” she says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter