Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Vertex buys Concert’s cystic fibrosis drug
Acquisition validates deuterated drug design and expands firm’s position in disease area
by Ann M. Thayer
March 6, 2017
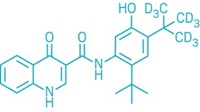
Vertex Pharmaceuticals will pay up to $250 million for Concert Pharmaceuticals’ cystic fibrosis drug CTP-656. Currently in Phase II clinical trials, the drug is a deuterated version of Vertex’s own compound ivacaftor, which it sells under the name Kalydeco.
Like ivacaftor, CTP-656 is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator. Substituting deuterium atoms for specific hydrogens in the structure generates a novel compound with altered pharmacokinetic properties. Notable is its longer half-life, which means that CTP-656 may need to be given only once per day versus two times daily for Kalydeco.
For Concert, the deal is a validation of its deuterium chemistry approach to drug design and an opportunity for CTP-656 to advance into late-stage development and commercialization. Vertex already leads the cystic fibrosis drug market with Kalydeco and Orkambi, a CFTR potentiator combination it launched in 2015. Sales of the two products, which are used by people with specific mutations in the CFTR gene, reached $1.7 billion in 2016.
Vertex will acquire rights to all of Concert’s other cystic fibrosis research and preclinical programs. Concert intends to use the money to help support itself through 2021, expand its pipeline, and advance its deuterated JAK 1/2 inhibitor CTP-543, now in Phase II testing against the autoimmune disease alopecia areata.
The deal brings at least three benefits to Vertex, Leerink stock analyst Geoffrey C. Porges told clients in a report. It removes a potential competitor and increases the company’s cystic fibrosis franchise with a new compound that can improve its existing therapy combinations and extend its intellectual property protection by at least five years.
Concert’s shareholders and regulators must still approve the sale of the drug to Vertex.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter