Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
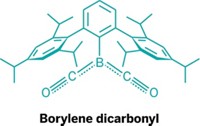
Borylenes nab nitrogen
Notoriously inert gas captured by a main-group element for the first time
by Bethany Halford
February 22, 2018
| A version of this story appeared in
Volume 96, Issue 9

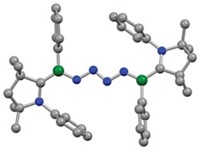
Boron has mastered masquerading as a metal. In recent years, compounds with the little p-block element have managed to latch onto small molecules, such as hydrogen and carbon monoxide, that chemists previously thought only metals could bind and split. Now, boron has gotten hold of the most stubborn small molecule, N2.
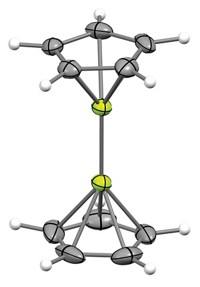
Researchers led by Holger Braunschweig of Julius Maximilian University Würzburg managed the feat by using a borylene—the boron version of a carbene—which features a monovalent boron, a lone pair of electrons, and an empty orbital. “The borylene mimics a very electron-rich transition metal,” Braunschweig explains. It donates its electron density to activate the stable N≡N bond (Science 2018, DOI: 10.1126/science.aaq1684).
“Nitrogen fixation by small molecules is among the most difficult tasks in chemistry,” says Suning Wang, an expert in organoboron chemistry at Ontario’s Queen’s University who was not involved in the research. The discovery, she says, “provides a new paradigm in nitrogen fixation and activation chemistry.”
Although the finding might someday help improve large-scale nitrogen-fixing reactions, such as the ammonia-making Haber-Bosch process, that goal, Braunschweig says, is a long way off. At the moment, the reaction, which requires two borylenes for every N2 molecule caught, isn’t catalytic.
“For years, low-valent main-group element derivatives were regarded as laboratory curiosities, only of interest for a restricted scientific community,” says Guy Bertrand, a University of California, San Diego, professor who also studies borylenes. “The fixation of N2 at a low coordinate boron center is a wonderful demonstration of the usefulness of fundamental research.”
Next, Braunschweig and colleagues plan to study the reaction’s mechanism. “Only when we know exactly how the compounds are being formed will we be able to optimize the conditions to make the reaction work better,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter