Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
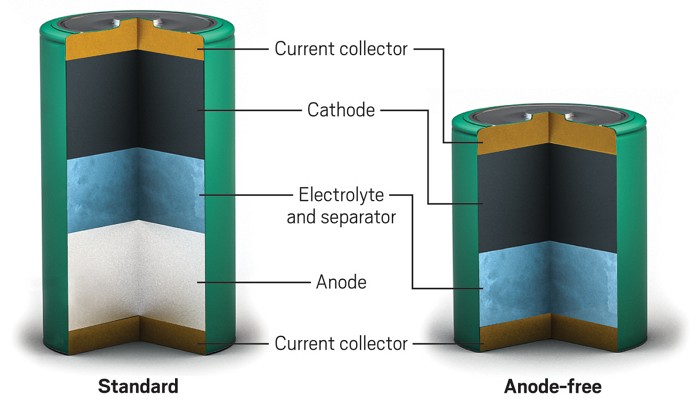
Zinc-ion batteries could reach higher energy densities by avoiding a traditional anode
Without a thick zinc metal anode, the batteries can be smaller and lighter
by Carolyn Wilke, special to C&EN
February 3, 2021
| A version of this story appeared in
Volume 99, Issue 5
Spurred by concerns over cost, safety, and sourcing of raw materials, researchers are on the hunt for alternatives to lithium-ion batteries. Now, scientists have developed a proof-of-concept, rechargeable zinc-ion battery that forgoes the standard zinc anode, giving it a relatively high energy density (Nano Lett. 2021, DOI: 10.1021/acs.nanolett.0c04519).
The disposable alkaline batteries used in many everyday electronics are based on zinc, but “researchers are making real progress in making these systems rechargeable,” says Marshall A. Schroeder, a materials engineer at the US Army Combat Capabilities Development Command Army Research Laboratory, who was not involved with the work.
Zinc, which is stable in air and compatible with aqueous electrolytes, presents a low-cost and potentially safer option for rechargeable batteries than lithium and sodium, which typically use flammable organic electrolytes. In many rechargeable zinc-based batteries, a thick zinc foil serves as the anode and source of zinc ions. “The problem is that you really only use a small amount of that zinc,” says study coauthor Husam N. Alshareef, a materials scientist at King Abdullah University of Science and Technology. The extra zinc hides inefficiencies in the battery’s charging and discharging and would also increase the battery’s cost.
So Alshareef and coauthor Yi Cui of Stanford University removed the zinc metal anode altogether. Instead, their cell preloads the cathode, made of manganese oxide, with zinc ions and contains zinc in the electrolyte. Without the excess zinc that was in the foil, the battery is lighter overall and can store more energy per unit weight. The researchers calculate that the battery’s energy density is 135 W·h·kg-1 compared with 81 W·h·kg-1 for a more typical zinc-ion battery in which the zinc anode makes up 20% of the battery’s weight.
During charging, metallic zinc forms on a copper current collector coated with carbon instead of depositing on a zinc anode. It’s a solution that looks deceptively simple, Alshareef says, but it took many attempts to find a coating that promotes the formation of a smooth layer of zinc, important in preventing needle-like dendrites that can short the battery.
“We have a very limited zinc amount in the whole anode-free battery,” says materials scientist Yunpei Zhu, who is part of the team at KAUST. But a minor amount of zinc is lost during every charging-discharging cycle, possibly due to undesirable reactions. “That means we are losing cycling stability,” he says. After 80 cycles, the prototype retains 68.2% of its original energy capacity.
But the team is already working to improve cycling stability. By tweaking the electrolyte chemistry, Zhu says, the researchers have made a newer version that loses only 5% capacity after 50 cycles.
This zinc-ion battery echoes earlier and ongoing efforts to make other types of anode-free batteries, Schroeder says. But despite years of research on sodium- and lithium-based systems, “progress has been relatively limited,” he says. Most cells haven’t exceeded 100 to 200 cycles, “so this work represents impressive progress in that context.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter