Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
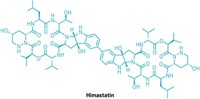
Biology inspires new route to himastatin
Dimeric natural product’s halves snapped together in final stage of synthesis
by Bethany Halford
March 25, 2022
| A version of this story appeared in
Volume 100, Issue 11

Himastatin, a natural product churned out by the bacterium Streptomyces himastatinicus, has an unusual dimeric structure built around a biaryl core. Chemists are interested in making the molecule because it has antibiotic activity, even though its structure doesn’t resemble other antibiotics. Seeking a route to build himastatin and provide quick access to diverse derivatives, chemists at the Massachusetts Institute of Technology developed a synthesis of the molecule that mimics nature’s biosynthesis. Instead of starting with the biaryl core and building outward, as other chemists have done when synthesizing himastatin, the MIT team, led by Mohammad Movassaghi and Bradley L. Pentelute, built the complex halves of the dimer and then used a one-electron oxidation to snap them together in the final stage of the synthesis. “All the work in this area concentrates on making that biaryl linkage at an early stage, but nature is doing it as the very last step. So it was natural for us to say, ‘Can we do the same thing?’ ” said Kyan A. D’Angelo, who recently completed his doctoral studies in Movassaghi’s lab and presented the work at ACS Spring 2022 last week. “This type of a late-stage, complex fragment assembly provides opportunities for rapid diversification,” Movassaghi told C&EN. The chemists use their synthetic approach to build derivatives of himastatin, including one with a fluorophore that could provide clues about himastatin’s antibiotic mechanism of action. The team recently published the synthesis in Science (2022, DOI: 10.1126/science.abm6509).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter