Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Chemists create chiral molecules using cobalt catalysts and a zap of electricity
Electrochemical C–H activation builds diverse compounds enantioselectively
by Bethany Halford
March 28, 2023

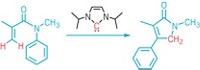
Metals like rhodium and palladium are C–H activation aces, but these metals are rare and expensive. For several years, chemists led by Lutz Ackermann at the Georg August University Göttingen have been using abundant, inexpensive cobalt catalysts for C–H activation reactions. The chemists also use electricity to drive the reactions, which means no redox reagents are required and the reactions’ only by-product is hydrogen. These researchers now report they can use this strategy to construct a broad range of molecules enantioselectively.
The chemists used the cobalt-catalyzed electrochemical reaction to prepare myriad molecules that are challenging to make enantioselectively, including spiro compounds (example shown), axially chiral compounds, and compounds with a stereogenic phosphorus atom. The chemists simply vary the cobalt’s chiral ligand depending on the molecule they wanted to make.
Ackermann presented the work at ACS Spring 2023, a meeting of the American Chemical Society, on Monday, during a talk in the Division of Organic Chemistry. It was also recently published in Science (2023, DOI: 10.1126/science.adg2866).
“This is an exciting new development in the growing area of asymmetric electrocatalysis,” said Song Lin, an electrochemistry researcher at Cornell University who was not involved in the work. Because the electrochemical reactions generate hydrogen, Lin said they present “a clean and potentially sustainable source of redox equivalents.”
The transformations could find use for creating drugs or agrochemicals, Ackermann said. But he told C&EN that the work “goes beyond simple molecular synthesis.” It could be used to build a decentralized hydrogen economy. He envisions small chemical synthesis setups, where, for example, a farmer could use a solar panel or a small wind turbine to power an electrochemical synthesis of a crop protection agent on site and simultaneously generate hydrogen that could be used for fuel.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter