Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Nanoantennas gather the power of light
Semiconductor-coated metal particles could harvest light energy for photovoltaics or catalysis
by Neil Savage, special to C&EN
March 24, 2022
| A version of this story appeared in
Volume 100, Issue 11

Combining the light-enhancing qualities of plasmonic materials with the charge-handling abilities of polymer semiconductors creates nanometer-scale antennas that might one day provide a new way to power certain chemical reactions.
Plasmons—oscillations of electromagnetic energy—can arise when light strikes the surface of certain metal nanoparticles. This phenomenon allows the nanoparticles to work as light harvesters, creating so-called hot charge carriers—excited pairs of electrons and their corresponding positively charged holes—that might be used in photochemical reactions, including for catalysis and as photovoltaics. Unfortunately, the carriers recombine quickly, sometimes as fast as in femtoseconds, turning the harvested optical energy into less useful heat.
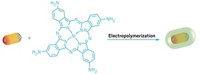
Chemical engineers Christy Landes and Stephan Link and their colleagues at Rice University found that coating the nanoparticles with a conductive polymer semiconductor allowed them to transfer the energy from the plasmons into the semiconductor, where the distance between electrons and holes was lengthened, increasing the carriers’ lifetimes to nanoseconds, long enough to do something useful (ACS Nano 2021, DOI:10.1021/acsnano.0c08982). Landes outlined the work Wednesday evening in a session of the Physical Chemistry Division at ACS Spring 2022, a meeting of the American Chemical Society.
“Nothing absorbs photons better per amount of material than a plasmonic metal nanoparticle,” Landes said. “But using that energy for something other than heat is the challenge and the polymer solves that problem.”
To create their nanoantennas, the team made gold nanorods that averaged about 85 nm long and 38 nm wide. They coated those with poly(nickel(II) 2,9,16,23-tetra(amino)-phthalocyanine), a photoconductive polymer. When they shone light on the nanoparticles, about 50% of the energy gathered in the plasmons was transferred to the polymer. The system’s properties can be altered by changing the nanoparticle’s size, shape, or material or by using a different polymer, Link says.
Nan Jiang, a chemist at the University of Illinois Chicago, praised the work. “This approach provides an unmatched way to harvest and transfer energy from light,” he said in an email. “I am sure that this approach will also open new avenues for triggering light-activated chemical reaction.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter