Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Nickel tamed in reagent toolkit
Collection of 10 bench-stable precatalysts will help chemists explore reactions with this low-cost metal
by Bethany Halford
March 22, 2022
| A version of this story appeared in
Volume 100, Issue 11

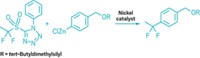
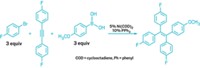
A new toolkit of 10 compounds that can be turned into nickel catalysts offers chemists a way to explore the metal as a catalyst for a range of reactions. Nickel(0) could be a less costly alternative to other metal catalysts, like palladium, but it tends to be finicky to work with. The stable-in-air reagents in the toolkit feature 1,5-cyclooctadiene (COD) as one ligand and either a quinone, a cyclopentadienone, a thiophene-S-oxide, or a fulvene as the other ligand. Chemists can screen the 10 so-called precatalysts to determine which works best for their transformation.
Nickel has a longstanding reputation as a difficult reagent. In his book Catalysis in Organic Chemistry, Paul Sabatier, the 1912 Nobel laureate in chemistry, compared a nickel catalyst to “a spirited horse, delicate, difficult to control, and incapable of sustainable work.” But Sabatier—and many chemists subsequently—have seen enormous potential for nickel: it can catalyze reactions such as cross-couplings just as palladium does, but at a fraction of the cost of that metal.
“Reactions that are widely used in pharma catalyzed by palladium are always at the mercy of fluctuating palladium prices,” said Keary M. Engle of Scripps Research in California, who led the work on the new nickel toolkit.
The problem with nickel is that the most commonly used Ni(0) precatalyst—Ni(COD)2—isn’t stable in air and has to be used in a glovebox with an inert atmosphere, which makes it impractical to use on larger scale. Engle’s group previously studied a bench-stable nickel precatalyst with COD and duroquinone ligands. “It didn’t have the breadth of reaction compatibility that we would like, so we wanted to expand that,” said Van T. Tran, who was a graduate student in Engle’s lab. This collection of 10 reagents allows chemists to mix and match compounds to find a precatalyst that has the desired reactivity.
The new toolkit democratizes the use of nickel, said Bill Morandi, an organic chemist at the Swiss Federal Institute of Technology (ETH), Zurich, who was not involved in the work. “The synthesis of bench-stable Ni(0) precatalysts is truly enabling for the discovery of new reactivity as well as downstream applications in both industrial and academic settings,” he said in an email.
A poster about the toolkit was presented on Tuesday in the Division of Organic Chemistry at ACS Spring 2022, a meeting of the American Chemical Society. And a preprint about the work, which has not been peer-reviewed, was published on ChemRxiv (2022, DOI: 10.26434/chemrxiv-2022-7zjvh). Engle told C&EN he is working with chemical suppliers to make the reagents widely available.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter