Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Sensing
Light-powered hydrogen sensor plays it cool
Sensitive chip uses palladium-decorated titanium dioxide to detect traces of flammable gas at room temperature
by Mark Peplow, special to C&EN
December 14, 2020

Even the faintest trace of hydrogen gas can spell trouble. In a chemical plant, it may auger a catastrophic industrial explosion; on a patient’s breath, it can signal various gastrointestinal problems.
Effective hydrogen sensors provide a vital way to detect this odorless, colorless gas. Now, researchers have unveiled a compact, light-activated hydrogen sensor that overcomes some key disadvantages of existing devices (ACS Sens. 2020, DOI: 10.1021/acssensors.0c01387).
In the laboratory, trace levels of hydrogen are typically measured using mass spectrometry techniques, but there is a growing demand for smaller, handheld sensors that can be used in a wider array of environments. Many of these portable sensors rely on changes in current or resistance when the gas undergoes catalytic oxidation on the surface of an electronic chip. But some of these devices require high temperatures of 150 °C or more to make their catalyst work, which raises operating costs and shortens sensor lifetime; other devices struggle to detect very low hydrogen concentrations of a few parts per million (ppm). In many sensors, gases such as nitrogen oxide can interfere with the device’s response, reducing its sensitivity to hydrogen. “They are designed for much higher hydrogen concentrations, near to explosive limits,” says Ylias M. Sabri, part of the team at RMIT University who developed the new sensor.

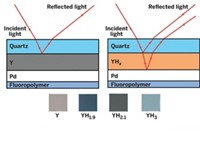
The team’s 1 cm long sensor chip is covered in a thin layer of titanium dioxide, shaped into a repeating pattern of micrometer-sized bumps that are highly efficient at absorbing light. These bumps are decorated with palladium nanoparticles, which capture and activate hydrogen molecules in a gas sample. Meanwhile, ultraviolet light from a light-emitting diode shines on the titanium dioxide to excite electrons—these react with oxygen in the air to generate oxygen anions, which combine with the captured hydrogen to form water. When the chip is operated at 9 V, this electrochemical process changes the current flowing through the device, which correlates to the concentration of hydrogen in the sample.
Crucially, the device operates at just 33 °C and can detect hydrogen at concentrations as low as 3.5 ppm. Exposure to much higher concentrations of hydrogen did not reduce its sensitivity in subsequent tests, avoiding a “memory effect” that has dogged other devices. And the sensor has virtually no sensitivity towards other gases, including CO2 and nitrogen oxide, although humid air reduced the sensor’s performance slightly. Sabri says that previous hydrogen-sensing chips have not been able to combine low temperature operation, high sensitivity, and high selectivity in this way.
“It’s an interesting approach,” says Bernard Dam of Delft University of Technology, who is developing hydrogen sensors based on optical fibers (Nat. Mater. 2019, DOI: 10.1038/s41563-019-0325-4). “In an electrochemical sensor you basically burn hydrogen, and the disadvantage has been that you need to heat it up to make it work. In this case, they don’t need to.” However, Dam wonders if the sensor’s palladium nanoparticles might become deactivated through exposure to carbon monoxide, which Sabri’s team has not yet checked.
Sabri thinks the sensor could be particularly useful in medical applications. Bacteria in our gut naturally produce enough hydrogen to leave around 10 ppm in our breath. But in people with gastrointestinal disorders, such as excessive bacterial growth in the small intestine, that can rise to 20 ppm. “This margin is very small, and that’s where our sensor is so good, because it can differentiate those concentrations,” Sabri says. His team now hopes to improve the device’s limit of detection to just 1 ppm, for example by increasing the surface area of its titanium dioxide layer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter