Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Sensing
Trifluoromethyl groups tug on cyclohexane
Molecule’s classic chair shape gets flattened as chemists position 6 of the polar moieties on one side of the compound
by Bethany Halford
July 25, 2020
| A version of this story appeared in
Volume 98, Issue 29

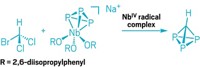
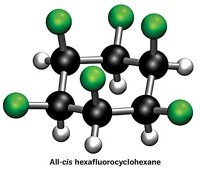
Researchers in David O’Hagan’s lab at the University of St. Andrews delighted chemists back in 2015 by making a cyclohexane with 6 all-cis fluorine substituents. Thanks to the molecule’s two sides—one with polar fluorines and the other with milquetoast hydrogens—it displayed surprising polarity, making it a “Janus face” molecule, a nod to the two-faced Roman god. Now O’Hagan’s lab and collaborators are at it again. This time, they’ve traded the relatively sleek fluorines for bulky trifluoromethyl groups (Angew. Chem., Int. Ed. 2020, DOI: 10.1002/anie.202008662). By hydrogenating hexatrifluoromethylbenzene, O’Hagan’s graduate student Cihang Yu made all-cis 1,2,3,4,5,6-hexakis(trifluoromethyl)cyclohexane. The reaction was tough. It required 14 days to make the desired material in just 13% yield, even with elevated temperature and pressure. A crystal structure of the material reveals that the classic chair conformation of cyclohexane gets flattened somewhat as the three axial trifluoromethyl groups splay because of their bulk. In the small family of all-cis hexakis substituted cyclohexanes that have been prepared to date, this molecule has the highest barrier to cyclohexane ring inversion, thanks to dramatic steric clashes between its trifluoromethyl groups. And while the molecule isn’t as polar as the all-cis hexafluorinated cyclohexane, its trifluoromethyl groups have enough pull to give it a mild affinity for chloride ions.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter