Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Imaging
Rhenium add-on tracks gold’s moves in cancer cell cultures
Fluorescent labels hint at why potential gold antitumor compounds behave differently around different cancer cell types
by Fernando Gomollón-Bel, special to C&EN
July 14, 2020

Gold compounds have shown promise as less toxic alternatives to widely used platinum anticancer drugs, such as cisplatin. Now, a team of researchers has designed new gold complexes with a luminescent tag, which allows scientist to follow the compounds’ paths to cells and better understand their mechanism of action (Inorg. Chem. 2020, DOI: 10.1021/acs.inorgchem.0c00813).
The only gold drug currently approved in the US, auranofin, treats rheumatoid arthritis. Other gold compounds are undergoing clinical trials as cancer treatments. “Gold binds very strongly to thioredoxin reductases, enzymes that are overexpressed in cancer cells,” says study coauthor M. Concepción Gimeno of Spain’s Institute for Chemical Synthesis and Homogeneous Catalysis. But the researchers didn’t understand how gold makes its way into cells and wanted to learn more.
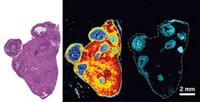
Building upon work looking at gold and rhenium complexes as trackable anticancer drugs, Gimeno, Vanesa Fernández-Moreira, and colleagues combined gold with a rhenium-bipyridine complex, a luminescent agent commonly used in cell imaging. The researchers can track the complexes’ biological trail using fluorescence microscopy, Gimeno says. They tested the compounds on human cervical cancer cells (HeLa) and lung cancer cells and found that the molecules bind to the cells’ membranes in different ways, suggesting membrane composition influences the molecules’ action mechanism. Showing such selectivity is a desirable property for an anticancer drug.
Combining therapeutic and diagnostic functionalities in one molecule, an approach known as theranostics, is a hot research topic, says Michael Coogan of Lancaster University, who was not involved in the work. This is the first study that hints at an explanation for why gold shows different toxicities to different types of cancer cells. Microscopy images suggest that this selectivity may be related to differences in the cell membranes, Coogan says.
The researchers also carried out mass spectrometry analyses to study the accumulation of the metals inside the cells. “Our results confirmed gold was being properly absorbed,” Gimeno says. Further experiments will investigate the uptake mechanism and shed light on the biological processes that the drug targets.
Studying the molecules in vivo will require adding different types of glowing tags to the gold complexes, because “the combination of cytotoxicity and luminescence would be lost in animal studies,” Coogan explains. “Such [experiments] would need near-infrared emitting complexes or another deeper-penetrating modality.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter