Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microscopy
Chemistry In Pictures
Chemistry in Pictures: Watch them grow!
by Manny I. Fox Morone
May 12, 2022
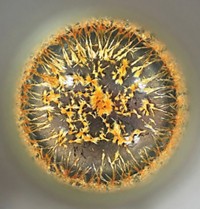
Jason Hein captured these ballooning crystals under a microscope in real-time. Molecules of sodium ammonium tartrate precipitate out of this solution fast enough and form big enough crystals—10 to 100 µm long—for him to see with an optical microscope. Hein, a professor at the University of British Columbia, was capturing these crystals’ growth as part of his lab’s larger project to automate organic chemistry processes. In this case the lab plans to use computer vision to track the progress of crystallization. Separately, sodium ammonium tartrate is a special compound in the history of chemistry: because its large asymmetric crystals are so big, Louis Pasteur was able to study them and manually separate them based on their handedness, similar to the mirror-image difference that distinguish right and left hands. That experiment that led him to discover molecular chirality 1848.
Submitted by Jason Hein
Do science. Take pictures. Win money. Enter our photo contest here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter