Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microscopy
Single catalyst molecules tracked in solution
Fluorescence microscopy traces Grubbs catalysts’ winding paths during polymerization
by Mark Peplow, special to C&EN
June 24, 2022

The catalysts that buzz around inside a reaction are rather like a swarm of midges—you know they’re there, but each one is so small and fast that they seem impossibly difficult to track in flight. Until now.
For the first time, researchers have measured the motion of individual catalyst molecules during a reaction (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c03566). Such insights could assist in the design of better catalysts, says Suzanne Blum of the University of California, Irvine, who led the work.
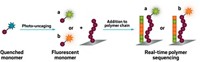
The research relied on superresolution fluorescence microscopy, which is already used to scrutinize the movements of single enzymes. But the technique has yet to tackle the challenges presented by imaging single molecules of chemical catalysts, Blum says. The team set their sights on a reaction that uses a ruthenium catalyst to add monomers to a growing polymer. Crucially, each catalyst molecule remains tethered to the end of a polymer chain, slowing the catalyst’s movement enough for reliable imaging.
During the experiment, the researchers interrupted the reaction once some polymer had formed and washed away any unreacted molecules. Then they added an extremely dilute solution of monomers tagged with fluorophores that briefly glow green when hit by blue light. These tagged monomers were incorporated into the polymer chains, right next to the ruthenium catalyst at the tip.
Giving the reaction a flash of blue light once every second provided regular updates on the positions of each polymer-bound fluorophore, and its adjacent catalyst. Meanwhile, unattached fluorophores still floating in solution moved around too quickly to register on the camera, so they did not muddle the images.
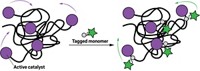
The microscope could pinpoint polymer-bound fluorophores to within 32 nm, enabling the team to calculate that about one-quarter of ruthenium catalysts were effectively stationary. A similar proportion moved around vigorously, traveling an average of 145 nm each second, while the remainder roamed more modestly.
This variation in movement may be due to the catalysts being more or less entangled in the spaghetti-like mass of polymer chains, which could also affect the catalysts’ access to monomers or solvent molecules. “The most important finding from the experiment is that during polymerization the catalysts exist in many different environments,” Blum says.
If that environment is too cramped or restricts the supply of monomers, the polymer strand will grow more slowly. That could in turn affect how long different polymer strands grow, a key factor in determining the polymer’s bulk properties.
“A polymer is a complex sample; it’s always a distribution of molecular weights and lengths, and now we start to understand where this is originating from,” says Johan Hofkens of KU Leuven, who works on single-molecule and superresolution microscopy. “I think it’s an interesting step forward to get this insight.”
Blum hopes that the method could help chemists to design catalysts that have more uniform motions during polymerization—for example, by tweaking their ligands so that they repel nearby polymer strands and ensure the catalysts can access a similar amount of monomers. This could produce more consistent chain lengths that improve the polymer’s material properties.
Her team is now applying the technique to other kinds of reactions. Not all reactions will be compatible with a fluorophore tag, Hofkens cautions. “But as long as you’re sure that you don’t disturb the process that you’re looking at, it’s a perfect technique, and it will find wider applications,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter