Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
Amyloids found in human cataracts
2-D infrared spectroscopy also detects amyloids in lenses without diagnosed cataracts
by Celia Arnaud
March 20, 2019
| A version of this story appeared in
Volume 97, Issue 12
Cataracts form when clear crystallin proteins in the eye lens aggregate into opaque deposits that scatter incoming light and cloud vision. Researchers had posited these aggregates contained amyloids, a type of fold some disease-related proteins, such as those associated with Alzheimer’s disease, adopt. But scientists hadn’t obtained experimental evidence to back up the hypothesis.
Now, graduate student Ariel M. Alperstein, chemistry professor Martin T. Zanni, and coworkers at the University of Wisconsin–Madison have detected amyloids in human cataracts using 2-D infrared spectroscopy (2D-IR) (Proc. Natl. Acad. Sci. U.S.A. 2019, DOI: 10.1073/pnas.1821534116). The researchers’ analysis indicates that many people could have these amyloids in their lenses and just not know it. Their findings suggest that the process of forming cataracts begins long before any cloudy vision manifests itself. Preventing amyloid formation or breaking up existing ones could help alleviate symptoms.
Most proteins in the eye lens are members of the crystallin family of proteins. “The lens crystallin proteins will form amyloids in a test tube, so it was always assumed that they would assemble into these same folded structure in the native tissue,” says Jason Gestwicki, an expert on protein misfolding who works at the University of California, San Francisco. Proving that idea was difficult, he says, because the usual methods for measuring amyloids, such as transmission electron microscopy (TEM), are not amenable to use in the lens.
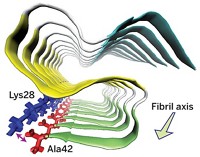
The Wisconsin team acquired 2D-IR spectra of lenses with visible cataracts, mature lenses with no visible cataracts, and juvenile lenses. The defining characteristic of amyloids is an extended β-sheet structure. The researchers could detect that structure in the 2D-IR spectra of amyloids through characteristic features including frequency shifts of cross peaks associated with an amide band of the spectra.
They detected amyloids at levels of about 5% on average in the cataract-containing lenses and up to 40% in specific locations. Interestingly, they also detected amyloids in mature lenses that had no visible signs of cataracts. Juvenile lenses didn’t contain discernible amyloids. The amyloid fibrils they found were much smaller than the ones that form in solution. Zanni speculates that the amyloids may be so small because a large fraction of eye proteins are chaperone proteins whose function is to prevent misfolding and aggregation.
“From the data it sounds like these are short amyloid fragments accompanied by a mix of protein fragments, UV-modified protein, and denatured proteins,” says Patrick C. A. van der Wel, an amyloid expert at the University of Groningen. “A big puzzle is that non-cataract aged lenses show similar damage. That leads to the question of why certain lenses with 5% amyloid structure and UV-damaged proteins do not have a cataract diagnosis while others do. What is the difference?”
The work “could be a significant step forward in our understanding of the molecular structures underlying age-related cataract,” says Justin L. P. Benesch, an amyloid expert at the University of Oxford. “Given the recent successes in finding small molecules that appear to reverse lens opacification, there are obvious experiments to be done to determine whether the amyloid in the eye lens is causative for cataract or an innocent bystander.”
Advertisement
“The Zanni group’s use of 2D-IR to probe for amyloid structures in the intact lens is both satisfying and important. This work finally links decades of careful biophysical work with the context of human disease, an important step in the understanding and treatment of any disorder,” UCSF’s Gestwicki says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter