Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
Making molecular movies of vision
Researchers use femtosecond pulses of light to watch what happens when light hits the eye
by Laura Howes
March 23, 2023
| A version of this story appeared in
Volume 101, Issue 10
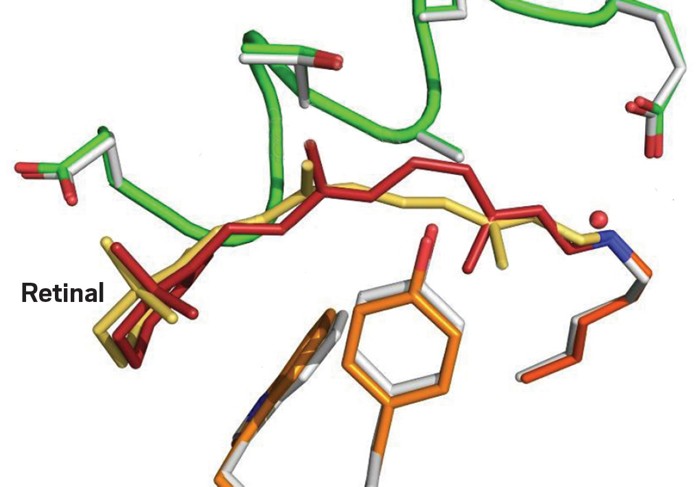
For your eyes to read these words, they need to detect light. When light hits your retina, it initiates a cascade of signals and changes that connect your eye to the brain. A team of researchers in Switzerland have now used super quick pulses of laser light to capture some of the very first steps of that process (Nature 2023, DOI: 10.1038/s41586-023-05863-6).
Inside the light detecting rod cells of the retina are light receptor proteins called rhodopsins. At the center of each rhodopsin protein is a small molecule, retinal. When light hits the retinal, that flips one double bond from a cis to trans conformation. That isomerization is just enough to trigger everything. But while researchers know the start and the end point, they haven’t been able to watch the switching process take place, until now. A team led by Valérie Panneels and Gebhard Schertler at the Paul Scherrer Institute in Switzerland have used X-ray free-electron lasers to take a series of snapshots using a technique called time-resolved serial femtosecond crystallography. In doing so, they’ve shown how retinal takes about one picosecond to switch to an all trans configuration via “aborted bicycle pedal” internal rotation. The isomerization breaks hydrogen bonds and van der Waals interactions with the protein, which then changes its conformation, starting the set of changes that result in a signal saying “here was light.”
There is more to uncover in the future, Panneels says. The team is now working to see what happens when the photon is first absorbed and also hope to watch how conformation changes propagate through the rhodopsin protein before activating the next steps in the light detection process.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter