Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
Structure of active ribonucleotide reductase solved with cryo-electron microscopy
Long-awaited image of elusive disordered tail helps scientists better understand the enzyme’s mechanism
by Celia Henry Arnaud
March 26, 2020
| A version of this story appeared in
Volume 98, Issue 12

Ribonucleotide reductases (RNRs) play a crucial role in cells as the only source of the building blocks needed for DNA replication and repair. They use radical-based chemistry to catalyze the conversion of ribonucleotides to deoxyribonucleotides. Because of this vital cellular function, RNRs are targets for cancer drugs, antibiotics, and antiviral drugs.
Now researchers have used cryo-electron microscopy to solve the first structure of an active form of an RNR.
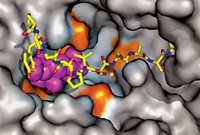
These complicated enzymes consist of two subunits, α and β. In the Escherichia colienzyme, both of these subunits are homodimers, and there are two active sites, one in each α subunit. Structures of the individual subunits have been reported before, but none of those structures resolved the catalytically essential disordered tails of the β subunit. Getting a structure of the intact complex has been difficult because interactions between the subunits are transient.
Former grad student Gyunghoon “Kenny” Kang, Alexander T. Taguchi, JoAnne Stubbe, and Catherine L. Drennan of the Massachusetts Institute of Technology have solved a structure of a class Ia RNR from E. coli at 3.6 Å resolution, including one of the usually disordered β tails (Science 2020, DOI: 10.1126/science.aba6794). The team captured an active form by replacing two amino acids, including the tyrosine where the radical chemistry starts. In the absence of an actual crystal structure, the RNR field has long used a computational model of the enzyme structure. That model depicts the structure as symmetric, but this new work reveals a marked asymmetry. One side of the enzyme is in a preturnover state, and the other side is in a postturnover state.
The structure shows one of the β tails reaching into the α subunit, where it forms one side of the substrate binding pocket. Assembly of the binding pocket also locks the proton-coupled electron transfer pathway into place. This pathway moves the radical from the initiation site in the β subunit to the active site in the α subunit, a distance of 32 Å.
“Unless the tail is in its designed binding pocket, it’s disordered. And that binding pocket is only established when everything is bound and ready to go,” Drennan says. “It’s perfectly designed so you can have order only when you’re ready for the radical.”
“The structure reveals a more intimate embrace of the two subunits than I had anticipated,” says J. Martin Bollinger Jr., a chemist at the Pennsylvania State University who studies another class of RNRs. (He was a graduate student with Stubbe in the late 1980s and early 1990s.) “The extensive penetration of the β tail into the α active site, which is essential to even constitute the substrate binding site, is nothing less than stunning,” Bollinger says.
For Stubbe, the structure is the culmination of decades of work using biochemical and spectroscopic methods to study RNRs. “Nobody would have predicted what we saw” in the β tail, Stubbe says. “But what we saw is consistent with all the stuff we’ve been doing for the past 20 years.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter