Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
X-rays show full-length P450 enzyme for first time
Structural details could help engineer new biocatalysts
by Laura Howes
May 29, 2020
| A version of this story appeared in
Volume 98, Issue 21

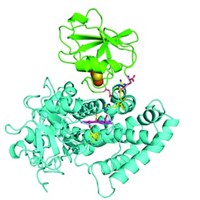
The first-ever structure of a full-length P450 enzyme has been solved. Cells use these enzymes to synthesize hormones and metabolize drugs. Chemists would like to engineer them to carry out reactions such as toxin degradation and the tricky oxidations needed to produce some pharmaceuticals—but this has been difficult to do without the full crystal structure.
“It is an outstanding achievement to get the full-length enzyme,” says Sabine L. Flitsch, a professor of chemistry biology at the University of Manchester who wasn’t involved in the work. Many groups, including her own, have tried to solve these structures because of their potential usefulness as biocatalysts.
The structure of the P450 enzyme called CYP116B46, which is made by a thermophilic bacterium, was solved by a team at Hubei University led by Rey-Ting Guo and Chun-Chi Chen (Nat. Commun. 2020, DOI: 10.1038/s41467-020-16500-5). Chen says that until now, researchers like herself and Guo were only able to refer to partial structures, which limited their ability to engineer the enzyme. “Now we have the chance to engineer the reductase domain and the electron transfer path,” she says.
P450 enzymes have been difficult to map because they are challenging to crystallize. CYP116B46 is relatively stable, and Chen and Guo were able to put it through the X-ray crystallography process. The protein is made from a heme domain and a reductase domain containing flavin mononucleotide (FMN); the two sections are linked by a ferredoxin domain that contains an iron-sulfur cluster.
Guo and Chen say the structure shows that in this enzyme, the FMN is close enough to the iron-sulfur cluster to enable direct electron transfer, but the heme is too far from the cluster to allow for it. Instead, they suggest that the electron is shuttled along by different polar residues in the protein. Flitsch says this might help explain the low activity of the bacterial enzyme.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter