Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New No Directions
Mesilla workshop highlights increasing evidence that reactive and mysterious HNO may be an important biomolecule
by Elizabeth K. Wilson
March 8, 2004
| A version of this story appeared in
Volume 82, Issue 10

In the 1980s, when nitric oxide's reputation suddenly shifted from that of noxious, polluting gas to vital biological molecule, researchers knew they'd only hit the tip of the iceberg.
The reactive diatomic radical that controls everything from heart health to erections begets a number of chemical offspring, including NO2, N2O3, and ONOO–. These NO species have their own nontrivial biological effects, some good, some bad. And in the ensuing years, researchers have become intimately acquainted with their ever-expanding biochemistry.
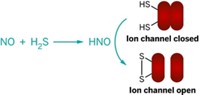
Very recently, however, a new player in NO research has emerged: HNO, an unusual, evanescent molecule whose biochemical potential has until now been largely ignored. In the past few years, a growing collection of chemists has come to believe that HNO, also known as nitroxyl, may play a significant role in biology and pharmacology, protecting the cardiovascular system, interacting with enzymes, and suggesting new drug possibilities. It can also cause DNA damage.
Although NO and HNO (and its deprotonated partner, NO–) appear to be closely related, their chemistries are quite different. NO likes to react with radicals; HNO prefers thiols. NO leads to increases in the important biochemical messenger cyclic guanosine monophosphate (cGMP), while HNO appears to increase levels of a different messenger, cyclic adenosine monophosphate. In fact, in biochemical experiments, the two are never seen carrying out the reactions of the other.
HNO is also a much slipperier target than NO. Because it dimerizes almost immediately, it can't be stored, and instead has to be produced on the spot with reactive reagents. And right now, its presence can only be detected indirectly, through characteristic downstream products.
Most controversial, there's still no evidence that it's endogenous to biological systems. However, researchers have their eye on a number of potential in vivo sources. And they've shown in the lab that HNO has powerful effects on some of the most important of biological molecules.
That, they say, makes HNO a molecule worth studying, and it's a field ripe for the picking.
"The chemistry by which HNO manifests itself pharmacologically is totally open," according to Jon M. Fukuto, who is an associate professor in the molecular and medical pharmacology department at the University of California, Los Angeles.
Fukuto was one of some two dozen researchers who discussed the biochemistry of NO derivatives at the annual Mesilla Chemistry Workshop, held last month in Mesilla, N.M. Modeled after the Telluride Workshop and the Gordon Conferences, the Mesilla workshop features small gatherings in an intimate setting, encouraging an unfettered exchange of ideas.
There, invited speakers discussed the chemistry of NO relatives such as nitrosothiols and peroxynitrite (ONOO–), formed by reaction of NO with the biologically ubiquitous superoxide radical (O2–). NO's interaction with enzymes and metals also featured prominently.
The conference also marked a first-ever gathering of HNO researchers to discuss what they call the "forgotten nitrogen oxide."
"This was probably the first time that quite a few of these people have been put together," said George Wang, chemistry professor at Ohio State University, and coorganizer of the Mesilla conference.
On the horizon, attendees reported, are new HNO donors. They also shared news about HNO's potentially protective effect on the cardiovascular system. And soon, they hope, will come methods for detecting evidence of HNO directly.
NO biology itself is still relatively young--it was only 15 years ago that researchers found that the molecule relaxes blood-vessel walls. In 1992, Science proclaimed NO "molecule of the year." And in 1998, three researchers who discovered its biological importance, including UCLA pharmacology professor Louis J. Ignarro, won the Nobel Prize in Physiology or Medicine.
NO is produced in organisms by a collection of heme-based enzymes known as nitric oxide synthases (NOS). They catalyze the hydrolysis of L-arginine, which produces NO and citrulline. NO then goes on to participate in a host of biological reactions, including binding with the heme component of the enzyme guanylate cyclase. That, in turn, stimulates the production of cGMP, leading to smooth-muscle relaxation.
BUT IT WAS WHILE STUDYING nitric oxide's myriad biological effects in the early 1990s that researchers began to turn up evidence for HNO. For example, Herbert T. Nagasawa, medicinal chemistry professor at the University of Minnesota, Twin Cities, found that HNO was produced from the reaction of thiols with nitrosothiols and that HNO can react with protein thiols to disrupt their actions.

Martin Feelisch, professor of medicine and biochemistry at Boston University School of Medicine, began to investigate the possibility that Angeli's salt (Na2N2O3), a compound that decomposes to produce HNO, causes vasorelaxation.
Several labs, including those of Ignarro, Fukuto, and Feelisch, proposed that HNO might even be generated by NOS.
"It seemed that we were tripping over HNO chemistry all the time," Fukuto said. "So we wondered if it was possible that HNO was made either as a precursor to or independent of NO."
And a few years ago, Dennis J. Stuehr, a biochemist in the department of immunology at the Lerner Research Institute in Cleveland, did further work with NOS, finding that it can produce HNO when it's made to react without a key cofactor, tetrahydrobiopterin.
As the reports have begun to accumulate, scientists are realizing that they have an intriguing, if still poorly understood, phenomenon on their hands. "We're at the same level now with HNO as at the beginning of the NO field 15 years ago," said Katrina M. Miranda, chemistry professor at the University of Arizona.
The primary reagent for producing HNO has been Angeli's salt, a highly oxidizing compound that readily decomposes to form HNO. Another, albeit less well-behaved, HNO donor is Piloty's acid, benzenesulfohydroxamic acid.
THESE COMPOUNDS have been used in many recent studies on HNO biochemistry. For example, Fukuto and his colleagues suspect that thiols are a major target for the electrophilic HNO. His group studied the effects of HNO on Ace1, a transcription factor in yeast studded with thiol groups. Normally, the thiols bind with copper to activate Ace1. But when Ace1 is exposed to Angeli's salt, it is inactivated, presumably because HNO has reacted with its thiol groups.
Some of the most well-publicized evidence for HNO's biological potential comes from heart studies by a group that includes conference attendees Feelisch, Miranda, Fukuto, and David A. Wink Jr., a biochemist at the National Cancer Institute in Bethesda, Md.

During a heart attack, a blockage cuts off blood flow to the heart in a process known as ischemia. But when the clogged heart is unblocked, blood surges into the tissue, which is called reperfusion. In reperfusion, the excess oxygen from the surging blood is reduced to reactive oxygen species, such as hydrogen peroxide, setting up a cascade of oxidative stress and leading to heart tissue death. "It's like being in a sinking ship and moving from one side to another," Wink said.
But while the researchers found that NO protects the heart from reperfusion damage, there was no question that HNO, when administered during reperfusion, made things worse.
They then hooked up with Johns Hopkins Medical Institution professors of medicine David A. Kass and Nazareno Paolocci. The group explored another phenomenon known as preconditioning, in which, paradoxically, minor ischemias make the heart better able to withstand a major heart attack.
They pretreated dog hearts with Angeli's salt, then subjected them to short bursts of ischemia. In the control group that received no pretreatment, 40% of the heart tissue died during reperfusion. However, in the hearts pretreated with Angeli's salt, surprisingly, only 15% of the tissue died off, and the heart's contraction ability increased. The group didn't see the same effect with NO donors [Proc. Natl. Acad. Sci. USA, 100, 5537 (2003)].
The suggestion that HNO donors might be useful as a new type of drug, possibly replacing nitroglycerin for treating heart failure, set off a wave of excitement and gave the field even more impetus.
"Just a year or two ago, those guys were pretty much in the wilderness," Stuehr said. "It's amazing how a little bit of medical data can energize people."
MEANWHILE, on the other end of the chemistry spectrum, theorists and physical chemists made a discovery that changed the whole landscape of HNO's biological potential. Research in the 1970s indicated that HNO was a relatively strong acid, deprotonating with ease. Back then, chemists in Germany and Canada performed pulse radiolysis experiments on aqueous NO, and concluded HNO had a pKa of 4.7.
That meant that under physiological conditions, the NO– form of nitroxyl would predominate. But this would react with NO to form N2O2–, and then N2O. Today, researchers know that there's enough NO in the body that if this were the correct picture, HNO wouldn't be significant. Clearly, there was a discrepancy between this and what they were seeing in reactions with HNO precursors.
Previously, researchers had assumed that, like most molecules, both ground-state NO– and HNO existed in the so-called singlet state. Molecular oxygen is one well-known exception to the singlet-ground-state rule, existing in the triplet state. Since NO– has the same electronic structure as O2, its ground state is now recognized as the triplet state. For HNO to give up a proton to become NO–, the molecule must change its spin state from singlet to triplet, a quantum mechanically forbidden transition.
UCLA chemistry professor Kendall N. Houk, who coorganized the Mesilla conference with Wang, showed with calculations that HNO should have a pKa of around 12. And Sergei V. Lymar, a physical chemist at Brookhaven National Laboratory, working with Vladimir Shafirovich at New York University, showed with flash photolysis experiments on Angeli's salt that the rate of HNO deprotonation in solution is very slow--its pKa is 11.5.
That changed the biological picture entirely. The finding that HNO predominates under physiological conditions made it possible to envision the molecule existing for sufficiently long periods to generate some of its own chemistry. In fact, the discovery clarified the then-strange results Wink and his colleagues were getting during their heart experiments. "HNO should have been converted right to NO, according to the old literature," Wink said.
In fact, NO and HNO biochemistries appear to be "orthogonal," said Miranda. Where there's NO chemistry, there's no evidence of HNO, and vice versa.
NO and HNO are not readily produced from each other, so the chances of a biological route to NO protonation are slim. But the researchers say arginine oxidation, reaction of S-nitrosothiols with thiols, or NOS could all possibly produce HNO.
Not everyone is convinced of the biochemical importance of HNO, however. Lymar argued, for example, that in some cases HNO might not be the source of the observed chemistry. Stocks of Angeli's salt are kept stable in an alkaline solution, he said. But HNO released at this pH quickly turns into NO–, which reacts with oxygen to form peroxynitrite. "It's my personal belief that there's a great deal of ambiguity in interpreting the effects of Angeli's salt in complex biochemical systems, and that these experiments are very susceptible to artifacts," he said.
And Joseph S. Beckman, director of the Environmental Health Sciences Center and professor of biochemistry at Oregon State University, Corvallis, is concerned about the strong reactivities of HNO donors. He believes that alternative mechanisms could be at work in the dilute concentrations of reactants in cells. "You need better markers of what HNO does, and what the products are," he said.
With that, the researchers agree. "If you really want to test this, you need better HNO donors," Stuehr said.
To that end, several groups described a wave of new HNO donors. A group of molecules known as primary amine NONOates, including isopropylamine NONOate (IPA/NO), have traditionally been used as NO donors. Recent work by Miranda, chemist Larry K. Keefer at the National Cancer Institute, and John P. Toscano, chemistry professor at Johns Hopkins University, shows that the NONOates can also be HNO donors, depending on the pH of their environment. S. Bruce King, chemistry professor at Wake Forest University, Winston-Salem, N.C., and Nagasawa have developed a series of HNO donors based on the chemistry of nitrosocarbonyls.
Advertisement
The key objective of HNO research, of course, is a molecular trap that will provide unequivocal proof that the molecule has been there. That probably will be achieved in the next five years, predicted Fukuto.
For example, Patrick J. Farmer, associate chemistry professor at UC Irvine, has trapped HNO with deoxymyoglobin [J. Am. Chem. Soc., 126, 1096 (2004)].
But regardless of whether HNO is actually found in the body, some scientists argue, the pharmacological potential of HNO donors has been established.
And no matter what their position, the researchers all agree there's an interesting future ahead for research on the simple molecule with the strange chemistry. "There's nothing else like it in biology," Fukuto said.
DAMNED BY IMPLICATION
Picking Apart Peroxynitrite's Biochemistry
T

EUROPEAN ORIGINS
HNO Producers Have A Long History
H
CONFERENCES
A Bit Of Chemistry In The Old Southwest
S







Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter