Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Science Concentrates
May 17, 2004
| A version of this story appeared in
Volume 82, Issue 20
Fullerenes' golden child
Scientists have assumed that collections of a few dozen gold atoms prefer to exist as relatively uninteresting space-filling clusters. But now comes a prediction that a molecule composed of 32 gold atoms should also be able to form an exotic golden fullerene [Angew. Chem. Int. Ed., 43, 2678 (2004)]. Chemist Mikael P. Johansson and colleagues at the University of Helsinki, in Finland, performed quantum mechanical calculations that take into account relativistic effects, such as increased inner electron mass, that occur in heavier atoms. These relativistic influences help make possible a stable Au32 cage structure, as shown. Like its fullerene cousin C60, Au32 would be icosahedral. But unlike C60, electrons on the surface of Au32 would be able to move about freely. The interior of the molecule would also be magnetically shielded, perhaps more strongly than any other known molecule. The authors envision numerous uses for golden fullerenes, including drug transport.
Ice can be more dangerous than water when it comes to the toxicity of environmental contaminants. Chemists at Masaryk University in the Czech Republic compared the fate of 2-chlorophenol and 4-chlorophenol--contaminants from the paper and pesticide industries--when exposed to ultraviolet light in either ice or water [Environ. Sci. Technol., 38, 2873 (2004)]. In water, both chlorophenols were converted within 30 minutes to dihydroxybenzenes, which are themselves not stable in sunlight. In ice, the chlorophenols lasted for up to 10 hours, and the major products were more stable dimers, such as the one shown. In addition, the ice-derived products, after prolonged irradiation, were more toxic to marine bacteria and rat liver cells than either the water photoproducts or the parent contaminants. Arctic ecosystems are a sink for anthropogenic pollutants, says Jana Klánová, one of the coauthors. The Masaryk researchers believe that ice catalyzes unique reactions, but the mechanisms remain unclear. They are now studying the photoproducts of other pollutants in ice.
Despite considerable interest in engineered nanoparticles, the study of fullerenes larger than C76 has proven to be a challenge for scientists. That's because these molecules are formed in low yield as hard-to-isolate by-products during combustion-based industrial production of C60 and C70. Using a linked zinc porphyrin dimer as a host molecule (shown), Takuzo Aida, Kentaro Tashiro, and Yoshiaki Shoji at the University of Tokyo have devised a way of extracting these rare fullerenes [J. Am. Chem. Soc., published online May 11, http://dx.doi.org/10.1021/ja0489947]. When added to a mixed fullerene solution, the porphyrin dimer will selectively sequester the larger fullerenes. This host-guest complex can then be isolated via chromatography on alumina, and the two molecules can be separated by treatment with 4,4´-bipyridine. Using this method, the group was able to start with a mixture containing 10% higher fullerenes and, in a single extraction, isolate a mixture consisting of 97% higher fullerenes. Changing the length of the hydrocarbon linkers or adding substituents to the porphyrins can alter the host's affinity for a particular higher fullerene.
The flavin-containing enzyme uridine 5´-diphosphate (UDP)-galactopyranose mutase could be a target for drugs against tuberculosis, according to a study of the enzyme's mechanism of action [Nat. Struct. Mol. Biol., 11, 549 (2004)]. The enzyme catalyzes the conversion of UDP-galactopyranose to UDP-galactofuranose, a key precursor to a cell-wall component of Mycobacterium tuberculosis. Research by chemistry professor Laura L. Kiessling and coworkers at the University of Wisconsin, Madison, indicates that the conversion begins with nucleophilic attack on the pyranose by the flavin in the form of a reduced anion. Pyranose-to-furanose isomerization then occurs via a flavin-derived iminium ion. This mode of flavoenzyme reactivity has not been observed before, Kiessling says. Compounds that can bind the enzyme and alter the flavin's nucleophilicity would have antimycobacterial activity, the researchers suggest.
A previously unidentified metabolic route to the oxidized form of nicotinamide adenine dinucleotide (NAD+)--a small-molecule coenzyme essential for life--has been discovered by Pawel Bieganowski and Charles Brenner of Dartmouth Medical School [Cell, 117, 495 (2004)]. The finding upends the conventional wisdom that tryptophan and niacin are the only precursors to NAD+ in yeast, humans, and other eukaryotes. The researchers observed that even without a gene required for all described NAD+ pathways, yeast still managed to produce the coenzyme. Upon further investigation, Bieganowski and Brenner identified enzymes and genes in both yeast and humans that could convert a different molecule, the vitamin nicotinamide riboside (shown), into NAD+. Previously, scientists thought this NAD+ pathway existed only in certain bacteria. Bieganowski and Brenner also found that milk is a source of the vitamin. The Dartmouth researchers think that nicotinamide riboside could be useful as a vitamin supplement and that the vitamin and genes required for its utilization may be important in certain types of cancer treatment.



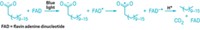


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter