Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Early-aging Disorders May Shed Light on Normal Aging
by SOPHIE L. ROVNER, C&EN WASHINGTON
August 23, 2004
| A version of this story appeared in
Volume 82, Issue 34
PREMATURE AGING
Symptoms that resemble the effects of early aging can occur as a result of several diseases, including Hutchinson-Gilford progeria and Werner’s syndromes. Researchers are studying such conditions in the hope that they can better understand normal aging. However, conclusions drawn from disorders associated with premature aging must be applied carefully because these diseases cause only some of the pathologies brought about by normal aging.
Patients with progeria syndrome show retarded growth and develop wrinkled skin, hair loss, and atherosclerosis when they are children. They usually die in their early teens.
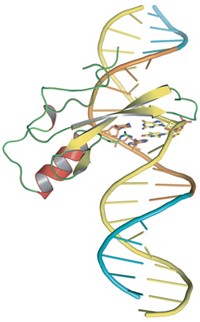
Independent teams led by Francis S. Collins, director of the National Human Genome Research Institute, and Colin L. Stewart, chief of the Cancer & Developmental Biology Laboratory of the National Cancer Institute, last year reported evidence that progeria is caused by mutations in the gene for lamin A, which appears to lead to abnormalities in nuclear membranes [Nature, 423, 293 and 298].
Those with Werner’s syndrome suffer from similar signs of aging and are more likely to get cancer than the general population. Patients typically live into their 40s or 50s.
The disorder arises from a mutation that inactivates the WRN gene. WRN codes for an enzyme that appears to be involved in DNA recombination, replication, and repair. When the gene is inactivated, cells undergo excessive DNA recombination and chromosomal aberrations. Werner’s syndrome may also interfere with the proper functioning of the telomeres that protect the ends of chromosomes.
Recent work backs up the link between failing DNA repair mechanisms and aging. Breaks in DNA can be repaired by replacing any missing DNA. Breaks can also be repaired by simply hooking the two loose ends together. As an organism ages, this mechanism becomes less efficient and more error-prone, leading to extensive deletions and rearrangements of the DNA, according to Vera Gorbunova, assistant professor of biology at University of Rochester in New York, and colleagues [Proc. Natl. Acad. Sci. USA, 101, 7624 (2004)]. The resulting mutations accumulate and contribute to the aging process and the development of cancer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter