Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Science Concentrates
January 3, 2005
| A version of this story appeared in
Volume 83, Issue 1
Cheaper, faster, more accurate DNA synthesis
Current methods for DNA synthesis are expensive, slow, and error prone. A newly reported microchip-based approach to gene synthesis could make this process cheaper, faster, and more accurate. George Church of Harvard Medical School, Xiaolian Gao of the University of Houston, and coworkers use DNA microchips (shown) to synthesize "construction" and "selection" oligonucleotides that are released from the chips, amplified by the polymerase chain reaction, and assembled into genes [Nature, 432, 1050 (2004)]. Tagged complementary selection oligos hybridize to the amplified construction oligos, fishing out and enriching those pieces that have been correctly synthesized. The selection process eliminates most oligonucleotides with mutations, resulting in a nearly ninefold reduction in synthesis errors. The researchers demonstrate the technology by synthesizing all 21 genes that encode the proteins in the small ribosomal subunit of Escherichia coli on a single microchip. The authors predict that using dense enough microchips could drive the cost of synthesizing oligonucleotides to as little as $1.00 per 20,000 bases. The technology is being commercialized by Houston-based Atactic Technologies, of which Gao is a founder.
Carbon nanotubes have been the rage of materials science because of their seemingly unlimited applications. But inorganic nanotubes could have diverse applications as well, including some that take advantage of the magnetic properties of inorganic materials. Researchers are just getting up to speed on how to make magnetic nanotubes, and in one of the latest efforts, electrical engineer Chongwu Zhou of the University of Southern California and his colleagues report the first single-crystalline magnetite (Fe3O4) nanotubes [J. Am. Chem. Soc., 127, 6 (2005)]. The team used a three-step process that begins by growing magnesium oxide nanowires followed by pulsed-laser deposition of a magnetite layer to give MgO/Fe3O4 core-shell nanowires, a method Zhou's group recently reported [Nano Lett., 4, 2151 (2004)]. Finally, the inner MgO core is etched out by a solution of ammonium sulfate, leaving the 30-nm-diameter Fe3O4 nanotubes. The magnetite tubes could serve as tunable nanofluidic channels or in magnetic data storage applications, Zhou says.
The Council of the University of Exeter, in England, decided on Dec. 20 to endorse a proposal to phase out chemistry degree programs and replace its existing School of Biological & Chemical Sciences with a new School of Biosciences. The proposal was carried overwhelmingly with 26 votes for, two against, and one abstention. The proposal, put forward by the university's vice chancellor, Steve Smith, is part of a package of measures designed to refocus the university's academic activity and reduce financial losses in departments, such as the chemistry department, that are running at a deficit (C&EN, Dec. 6, 2004, page 5). "The university will emerge in a much stronger position as a result of these changes," Smith says. "Our priority now is to make these changes and to ensure that we do the best for our staff and students." The U.K.'s Royal Society of Chemistry responded to the council's decision with a statement deploring the closure of the chemistry department. It says the decision represents an appalling consequence of underfunding of science and could set a precedent for other U.K. universities.
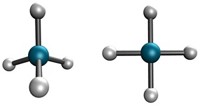
A new hypothesis on the mechanism of asthmatic inflammation has led to an ozone-scavenging compound that prevents bronchial obstruction in asthmatic rats. The idea was sparked by the surprising earlier finding by a Scripps Research Institute group that ozone can be produced by white blood cells during inflammatory processes as part of the body's defense against pathogens [Science, 298, 2195 (2002)]. This finding led Ehud Keinan and coworkers at Technion--Israel Institute of Technology, in Haifa, to speculate that the pulmonary inflammation that occurs in asthma might be caused by ozone production by white blood cells in lungs--and that inhalation of electron-rich olefins, which are known ozone scavengers, might therefore have antiasthmatic effects. In experiments in rats, they now find that inhalation of such a compound, limonene (shown), causes a significant improvement in asthmatic symptoms, compared to controls [Bioorg. Med. Chem., 13, 557 (2005)]. "It is an important finding," comments asthma specialist Nicola A. Hanania of Baylor College of Medicine, Houston, but "more studies, including human studies, will need to be done" to confirm the results.
An accurate theoretical description of the reaction of a hydrogen atom with methane reflects the increasing power of modern theoretical and computational chemistry. Previously, accurate quantum dynamics calculations could be performed only for simple gas-phase chemical reactions involving four or fewer atoms. Now, Uwe Manthe of the University of Bielefeld, in Germany, and coworkers report a full-dimensional quantum dynamics simulation of the six-atom reaction H + CH4 → H2 + CH3 on an accurate ab initio potential energy surface [Science, 306, 2227 (2004)]. The thermal rate constants predicted by this simulation fall within the range of the significantly varied experimental rate constants previously measured for this reaction and are expected to be as accurate as the best experimental measurements. "This achievement opens up the possibility of predicting rate constants accurately for many reactions of chemical interest," including ones involved in atmospheric and combustion chemistry, comments David C. Clary of the University of Oxford.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter