Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Solid Has the Most Spacious Pores
Chromium terephthalate-based material offers lots of room for storing molecules
by Ron Dagani
September 26, 2005
| A version of this story appeared in
Volume 83, Issue 39

NANOMATERIALS
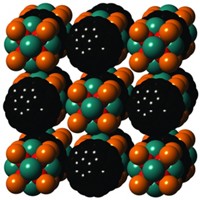
One could say that mil-101 is mostly nothing, but that's saying a lot: MIL-101 is a new, unusually porous material whose unit cell has an unprecedented volume of about 702,000 Å3, meaning that the solid is about 90% empty space once the solvent molecules normally filling its pores are removed. It also boasts pores that are 29 or 34 Å across and an internal surface area of 5,900 m2/g (Science 2005, 309, 2040).
"A tablespoon of MIL-101 has the surface area of a half-dozen football fields, or about seven times the area of the most catalytically effective zeolites," notes a Science commentary authored by professors Joseph T. Hupp and Kenneth R. Poeppelmeier of Northwestern University's chemistry department.
For comparison, cloverite has held the record since 1991 for the largest pores (close to 30 Å across), but its cell volume is only about 125,000 Å3.
Scientists are interested in highly porous materials for use in catalysis, separations, gas storage, sensors, and other applications. And MIL-101 appears to be a promising candidate for some of these uses, according to its discoverers--a team led by solid-state scientist Gérard Férey of the Lavoisier Institute at the University of Versailles, in France.
MIL-101--the letters stand for Matérial Institut Lavoisier--consists of inorganic chromium clusters linked by organic moieties. The key building block is a tetrahedral supercluster consisting of four smaller clusters (triads of oxochromium octahedra) that are held together by terephthalate (1,4-benzenedicarboxylate) groups. These superclusters form an extended framework containing cages of two different sizes.
The solid was prepared by heating terephthalic acid, chromium nitrate, hydrofluoric acid, and water at 220 °C for eight hours.
For a complex material having very large cells and pores like MIL-101, Férey explains, it's usually not possible to figure out the structure by using conventional single-crystal X-ray methods because the material is available only as a microcrystalline powder. So, with computer scientist Caroline Mellot-Draznieks, his team developed a computer program that provides structural information and simulated powder X-ray diffraction patterns for the architectures most likely to form (based on their energetic stability). By comparing the experimental powder X-ray pattern obtained for MIL-101 to the simulated patterns, the researchers found a match.
Férey is interested in using the pores of materials like MIL-101 as nanoreservoirs and nanoreactors. By introducing "nano-objects" into the pores, one could make a variety of nanomaterials consisting of uniform particles 2 to 3 nm in size. Larger species could be segregated in the large cages; smaller species, in the small cages. This approach could lead to new types of multifunctional nanomaterials.
The French team has already begun to explore some of these possibilities by filling MIL-101's pores with species as diverse as tungsten polyoxoanions, hydrogen gas, semiconducting zinc sulfide nanoparticles, and ibuprofen.
"It is the very beginning," Férey tells C&EN, "and the only limits, besides steric hindrance of the guests, are those of our imagination."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter