Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Mistic Boosts Protein Yields
Discovery allows eukaryotic membrane proteins to be made in high yields
by Amanda Yarnell
February 28, 2005
| A version of this story appeared in
Volume 83, Issue 9

BIOCHEMISTRY
A novel bacterial membrane protein can be used to produce large quantities of other tough-to-obtain membrane proteins, according to scientists at Salk Institute, San Diego [Science, 307, 1317 (2005)].
The discovery by Salk structural biologists Senyon Choe, Roland Riek, Tarmo P. Rooslid, and coworkers "opens new avenues around traditional obstacles in the study of integral membrane proteins, particularly those of eukaryotic origin." Such membrane proteins are thought to account for nearly a third of eukaryotic genomes and play central roles in a wide range of cellular processes.
It's become routine to determine the structure of soluble proteins, but membrane proteins are another story, the authors write. These proteins are notoriously difficult to crystallize. In addition, it's difficult to produce properly folded membrane proteins in high yields.
At the root of this production problem, the researchers continue, is the fact that eukaryotic membrane proteins often can't be overproduced in bacteria, the workhorses of recombinant protein production. The bacterial targeting machinery may fail to deliver the eukaryotic membrane protein to the lipid bilayer, or production of the eukaryotic membrane protein may kill the bacteria by overloading the machinery they use to insert their own membrane proteins into the lipid bilayer.
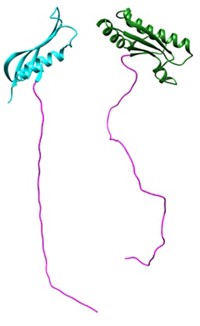
Now, the Salk team has discovered a small, unusual bacterial protein--dubbed MISTIC (Membrane Integrating Sequence for Translation of Integral Membrane Protein Constructs)--that promises to ease overproduction of eukaryotic membrane proteins in bacteria. Unlike typical membrane proteins, MISTIC readily folds into the lipid bilayer without the help of the targeting machinery that cells usually rely on to insert membrane proteins into the membrane.
Choe and Riek hypothesized that they might be able to exploit MISTIC to target another protein to the bacterial membrane. Indeed, they found that eukaryotic membrane proteins can be overproduced in bacterial cell membranes when expressed as MISTIC fusion proteins. So far, the Salk team has used MISTIC to produce nearly 20 different functional eukaryotic membrane proteins in bacteria, with yields of around 1 mg of purified protein from 1L of culture.
By combining a novel set of two-dimensional nuclear magnetic resonance spectroscopy experiments and electron paramagnetic resonance experiments using site-directed spin labels, the authors have found that MISTIC is a bundle of four ∝-helices with a polar lipid-facing surface.
They don't yet know exactly how MISTIC assists the production of other membrane proteins. Because MISTIC is surprisingly hydrophilic for a membrane protein, the authors suggest that it might be produced in the cytoplasm as a soluble polypeptide, and that it then spontaneously integrates itself into the lipid bilayer. MISTIC's integration may cause its eukaryotic membrane protein cargo to be positioned such that it spontaneously folds into its native, membrane-inserted conformation, they suggest.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter