Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Recalibrating the Clinic
High-tech tools and streamlined business processes are making their way to the far reaches of the drug development pipeline
by Rick Mullin, C&EN Northeast News Bureau
February 28, 2005
| A version of this story appeared in
Volume 83, Issue 9

Late last year, Sir Tom McKillop, chief executive officer of AstraZeneca, decreed a shake-up in drug development and clinical management at the drug firm. McKillop cited bad news from the Food & Drug Administration on its blood-thinning drug Exanta and its cancer treatment Iressa as the impetus for assigning Senior Vice President John Patterson to a new board-level post overseeing drug development.
McKillop charged Patterson with implementing substantial changes in clinical organization and processes and with improving regulatory capabilities and interactions with regulatory authorities.
There was more than enough bad news about drugs at the end of 2004, however, and McKillop's move was only the most public glimpse at the wave of reorganization that has already moved through discovery and early-stage development at large drug firms and is now building momentum in clinical management.
With that wave comes a raft of technologies and methodologies. Major pharmaceutical makers say they are starting to put genomics, statistical analysis, imaging, automated data management, and information technology (IT)--the very tools used to jump-start discovery in recent years--to work on expediting the costly final phases of bringing a drug to market. Several large drug companies have made major moves along these lines in the past year--some in recent weeks.
Executives at these companies claim that the new technology heralds a fundamental change in the science applied in the clinic. They also see it enabling change in work processes and management. Systems biology and biomarkers are being turned on patient populations as well as drug candidates, they say, in order to better design trials and predict outcomes.
Large pharmaceutical firms believe that just as "post genomic" tools helped zero in on winning compounds in discovery, the quest for a better understanding of data within the context of disease mechanisms will enable smaller, more targeted clinical trials with a higher success rate.
Change does not come easily to drug companies saddled with monolithic clinical organizations that for decades supported a high-volume regime designed to churn out blockbuster drugs. But the steep drop-off in late-stage drug candidates in recent years has led to speculation that the blockbuster model is played out and has called into question the value of compounds that are currently ramping up into development.
IDEALLY, technological advances will merge with interdisciplinary science and enhanced knowledge of disease to redirect the clinic toward more genetically targeted therapeutics, ultimately boosting success rates. Both the National Institutes of Health and FDA have launched programs--the Roadmap for Medical Research and the Critical Path to New Medical Products, respectively--focused on enhancing their own protocols and guiding the industry in efforts to improve clinical trials.
Aside from some cutting-edge science, however, much of the change under way in clinical management is fairly prosaic in nature. In fact, it can be called business process reengineering. This outsourcing- and IT-intensive tool for boosting efficiency came into play a few years ago in drug discovery--about five years after most other industries, including chemical manufacturing, finished with it--and is now being wielded in later stage drug development.
The four major drug companies interviewed for this article claim that clinical data management and IT are going through some level of conversion. All have new strategies for employing clinical research organizations, and clinical trials at the four firms are consolidating and shifting to regions such as Central Europe that offer cost and quality advantages over the U.S. and Western Europe. Each company claims that it is also weaving systems biology into the process (C&EN, Feb. 14, page 47).
All of this is happening as drug safety concerns place clinical studies under increased public scrutiny. Company sources agree that public pressure, which has already led to wider access to study results on the Internet, will impact clinical management and postmarketing surveillance of drugs and may ultimately affect the protocol and cost for trials.
At Wyeth, the rationale for restructuring clinical management is the increasing number of compounds entering the pipeline as a result of an aggressive push to launch new chemical entities (NCEs). "There is a wave of work coming our way," says Robert T. Maguire, chief of operations for clinical development at Wyeth Research. Clinical pharmacology--Phase I trials--has already made changes in order to keep up with discovery, and Phase II and III are next, he says.
"The fundamental question," according to Ira C. Spector, vice president of clinical trials at Wyeth, "is, 'What would you do if you knew that the number of compounds coming into clinical development was going to quadruple?' That is the situation we were facing five years ago."
The surge in new compounds resulted largely from goals for drug discovery and development set three years ago by Robert R. Ruffolo Jr., president of Wyeth Research. Through a series of efficiency improvements, Ruffolo said Wyeth would introduce 12 new drugs to early-stage development per year, up from an average of about three. In addition, the company would file eight Investigational New Drug (IND) filings per year, up from two; begin Phase III trials on three new candidates per year; and market two new drugs each year. Wyeth hit the mark on early-stage development in 2001 and Phase III trials in 2004, and the firm expects to file two New Drug Applications (NDAs) in 2006.
Much of the success of Ruffolo's program stems from classic business process redesign, on which Wyeth worked with consultants at Accenture (C&EN, Feb. 16, 2004, page 26). The company outsourced clinical trial data management to Accenture, in much the same way that firms like DuPont outsourced their computer infrastructure to consultants nearly 10 years ago. Wyeth also worked with Accenture in realigning work done in the clinic.
Maguire says the key organizational change was a shift from alignment of science, research, and trial management within specific therapeutic groups to a centralized clinical management organization in which specialists in therapeutic areas can communicate and share ideas and resources. The change was implemented last year.

WYETH BEGAN to address IT throughout the organization five years ago, Maguire says, and has succeeded in implementing an automated data retrieval network and an Internet-based system for managing trip reports from field monitors.
Not only did Wyeth have to integrate all the software--there is still no off-the-shelf clinical trial management IT system on the market--it had to do so without the benefit of programming standards, which are only now being established for clinical data.
"We had to decide whether to wait or go it alone, running the risk that what we've built may at some point become obsolete," Spector says. He notes that Wyeth's head of biostatistics and clinical technology, Jerald S. Schindler, is on the steering committee at the Clinical Data Interchange Standards Consortium (CDISC), the lead standards group in this area. "We have evolved with the standard and to some extent influenced it."
Wyeth is starting to measure its progress on reengineering clinical trials. According to Maguire, the company reduced the time from finalizing a study protocol to the first patient visit by 33% from 2003 to 2004. Over that period, there was also a 42% reduction in time from the last patient visit to "database lock," a 24% reduction in time from database lock to finalizing key statistics, and a 41% reduction in time from database lock to final clinical report.
Wyeth has also been channeling systems biology tools into the clinic. Last September, the company merged two existing divisions focused on discovery--experimental medicine and discovery medicine--into a new translational research group under Frank S. Walsh, executive vice president and head of discovery. According to Steven J. Projan, vice president for protein technologies, the group is doing a lot of early-stage clinical work.
Projan says the translational science group's main task in the clinic is to apply genomic methodologies and predictive techniques to distinguish one patient from another prior to treatment. The merger of the two discovery groups, he says, will make more routine what had been an ad hoc effort to employ biomarkers in an assay of safety and efficacy in the clinic.
The approach is paying off--Wyeth was the first to file a voluntary genomic data submission with FDA. The filing was based on blood samples that could predict patient response in a clinical study of AN-1792, a vaccine for Alzheimer's disease that the company was working on with Elan Corp. The project was stopped when 18 people developed encephalitis. Projan says the pharmacogenomics data will be useful on work with future vaccine candidates.
He says that while systems biology techniques will likely slow down the process of bringing candidates into Phase I trials, it will expedite Phase III trials. Projan admits that proteomics and metabolomics are emerging disciplines that are expensive and not fully reproducible in the clinic. "But they are very powerful," he says. "They have an excellent potential for finding disease and toxicity markers. They ain't cheap, but nothing is more expensive than running a Phase III clinical trial."
According to Projan, translational science is shaking up the staid routine of a large corporate organization that, given the nature of new drugs in development, must change. "If you ask whether we discovery types are dragging clinical research kicking and screaming into the 21st century, I would answer yes," he says.
PFIZER HAS also been working on aligning discovery, development, and commercial divisions in order to increase the survival rate of compounds moving through the pipeline, according to John E. Arrowsmith, executive director of global R&D project management. "Our mantra at the moment is productivity," he says.
As part of the integration of Warner-Lambert and Pharmacia, Pfizer has taken a close look at where to align resources. The company has also launched an attrition task force to examine where candidates die and determine the cause of death.
Like Wyeth, Pfizer has set aggressive goals for bringing drugs to market--the most prominent is to file 20 NDAs over a five-year period ending next year. With five NDAs filed last year, for a total of 12 since 2001, Arrowsmith says the company is on track to hit that target. Pfizer also ran first clinical tests of compounds in humans every two weeks and launched a Phase II study every 24 days in 2004. There are 142 NCEs in the pipeline at Pfizer and about 222 ongoing projects. "We are filling up the pipeline," Arrowsmith says. "Now, it is all about how to execute."
He says Pfizer is bringing new science into the clinic. "There is a big investment in high-throughput methods, but there are also certain investments we need to make in new science, like biomarkers, that are truly indicative of long-term efficacy," Arrowsmith says.
Managing development costs is also a matter of better organization. "We are moving away from having separate zones for exploratory development and full development and other zones for marketing," he says. "There are levels of expertise in each of these areas, but there is a level of vertical integration that aligns with the product, not with the organization. Over the last year, we have been moving in this direction."
STATISTICAL ANALYSIS is also being overhauled at Pfizer, according to Diane K. Jorkasky, vice president of global clinical sciences. Here, too, routines are being disrupted. "There are tried-and-true old areas that for some people are immutable, such as doing statistical clinical trials as first proof of concept where you have three or four dose levels, each compared to a placebo group," Jorkasky says. "These trials are very similar in design to the large Phase III trials. They are like 'mini-me' Phase IIIs, but they are big enough to cost a lot of money."
Instead, Jorkasky says, Bayesian statistics, a regimen designed to account for uncertainties inherent in model selection, can be employed to significantly reduce the number of patients needed while just as significantly increasing the number of dosage levels tested in order to get a good idea early on of the correct dosing for a full trial.
Statistical modeling can be combined with the use of biomarkers to assess the response curve as dosage increases, she says. "If you reduce 320 patients to 100, you save a lot of money, a lot of time, and a lot of the heartache that comes when you find out later you don't have the right dose," Jorkasky says.
Advertisement
Pfizer is also stepping up computer modeling in order to run in silico tests on molecules to predetermine dosage and fine-tune the design of clinical trials. The company has installed a central data repository and earlier this month hooked it up to a computer grid in which a network of personal computers adds horsepower to the processing.
"Each person running a mathematical simulation now uses the equivalent of 200 high-end computers," Jorkasky says. Clinicians can start to look at whether their mathematical assumptions and the model are correct before running the whole model, she says.
"By coupling math simulation, modeling, and pharmacokinetic data with a biomarker in an environment with central data access on an extremely rapid high-end computer, you can start to run your clinical trial in silico," Jorkasky says. "We've done this prior to having all this neat-o technology, but it was like trying to get from here to Pittsburgh in a Model T Ford. Now, we've trapped this together, and it's like blast-off. We're talking warp speed."
Jorkasky notes that grid computing has been used for years in mathematics, academic science labs, and even in drug discovery, but that it is just catching on now in clinical trial planning and management. Like Projan at Wyeth, she sees clinical managers having little choice but to adopt technologies that are already at work earlier in the pipeline. "There was a bit of lethargy, but things have been kick-started by the number of compounds coming through," Jorkasky says.
DRIVING THE revolution in statistical analysis, Jorkasky says, is a change in drug development targets. For decades, drug development focused on targets such as seven-transmembrane receptors that were readily available to investigation. A "ram-it-through paradigm" in clinical trials readily produced beta-blockers, H2 blockers, nonsedating antihistamines, and other big classes of drugs. "Now, we're looking at kinases, things that are intranuclear. You get into things like agonists," she says. "This changes the whole face of the portfolio and the ways you have to do things."
Change is under way at Roche, beginning with IT, according to Beat E. Widler, global head of clinical quality assurance. "In the next couple of years, all our clinical trials will be managed through electronic data capture," he says. "That will cut down the turnaround time between patient visit and availability of data. It will also allow us to use data generated on performance and efficiency to fine-tune the system."
Widler says clinical researchers with new IT tools will become more proactive in setting up trials and guiding automated systems to spot errors or missing information.
Roche has also begun partnering with two contract research organizations rather than spot contracting with a wider pool of CROs. "This cuts down on preparation time," Widler says. "When you work with a CRO, it is absolutely essential that they understand your internal processes and that you know theirs. If you have a long-term relationship, you do not need to explore this every time." Under the new arrangement, he says, Roche can outsource entire programs to one of its partners, rather than contract for assistance on an individual trial basis.
The company is also expanding globally, doing more of its trials in Latin America, China, and Eastern Europe. "Cost is not really the driver," Widler says. "If you want to get quality, you pay the price." The diversity of patient populations and the availability of skilled clinicians are what make trials outside the U.S. and Western Europe of interest.
Quality assurance is an overriding theme in improving clinical management organizations, Widler says. Roche is drawing heavily on the quality and risk management oversight procedures that have been employed for years in manufacturing, he says. The development group is also borrowing a practice from business management--a staff member has been assigned the role of ethics ombudsman. "There is a recognition that clinical has a management element beyond research," Widler says.
At AstraZeneca, Glenn J. Gormley, vice president of clinical development, says the firm's moves to improve clinical management have been in the works for months and are not a direct response to the bad news about Exanta and Iressa.
"We have been aware of the need to increase the efficiency and productivity of R&D for a long time. Over the last year, we have had some disappointments, and it has allowed us to step back and ask if we have the accountability and resources where we need them to be." This, he says, will be Patterson's guiding principle in the months ahead.
The company also hopes to gain efficiency by implementing an Internet-based data capture system, Gormley says. Like Wyeth, AstraZeneca is concerned about the lack of standards but is working with the industry and regulators on keeping its system in compliance with data protocol regulations as they emerge.
"ASTRAZENECA has had private discussions with FDA about systems we'd like to adopt," he says. "Those may get into more confidential discussions because the systems we are developing, we believe, provide some competitive edge. But there is need for a broader public discussion around this issue. And that is taking place," he says, pointing to the CDISC standards body and others.
Beyond straightforward efficiency moves, including the use of CROs to supplement in-house capabilities, there is an understanding at AstraZeneca that better assessment of drug candidates using systems biology tools is key to making early-stage pipelines pay off, Gormley says.
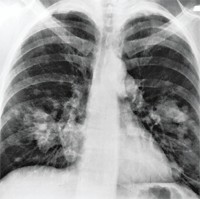
"We begin to ask if there are subpopulations to the groups we've looked at that will benefit from a drug mechanism and to tailor developments for that group," Gormley says. He adds, for example, that data on Iressa, which failed in clinical trials to prolong the lives of patients with advanced lung cancer, may ultimately result in a retargeting of the drug to a certain group of lung cancer patients.
"The challenge for the industry is to take the pipelines we have and identify the best potential candidates in that pipeline early," Gormley says. "If we can begin to identify the right drugs very early in development, using various biomarkers and surrogate end points, we can have a more efficient evaluation of those drugs, and the pipelines will not be seen as running dry. I think we do have robust pipelines if we develop them in the right way."








Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter