Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
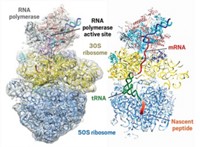
Biological Chemistry
Shedding Light On DNA Replication
Mechanistic studies fill holes in understanding of various aspects of DNA replication
by Celia Henry Arnaud
March 27, 2006
| A version of this story appeared in
Volume 84, Issue 13

Undergraduate biology students could be forgiven for thinking that scientists have DNA replication all figured out. After all, textbooks present the models of replication so matter-of-factly that it's easy to forget that many details are understood poorly, if at all. Recently published research sheds light on some of the gaps in our understanding of this key cellular process.
During replication, the DNA must be unwound and separated into its two strands by an enzyme known as helicase. Each of the strands is then copied separately. DNA polymerase, the enzyme that synthesizes new DNA, can't start copying DNA on its own. It requires short pieces of RNA known as primers to jump-start the process.
One of the challenges in DNA replication is keeping the two strands in sync. Because DNA can be copied only in the 5′ to 3′ direction, the correctly oriented strand, known as the leading strand, can be copied continuously as DNA polymerase just chugs along behind the helicase.
Things aren't so speedy for the so-called lagging strand, which is oriented in the opposite direction. The lagging-strand DNA must form a loop so that the DNA polymerase can copy it in the correct direction. Each time a loop is released and a new one is formed, a new primer must be synthesized and laid down, thereby slowing down replication. The newly copied pieces are later stitched together.
Biologists have long wondered what keeps the leading strand from getting too far ahead. Complicated models have been proposed to explain how the two strands keep pace with each other, but Antoine van Oijen, an assistant professor in the department of biological chemistry and molecular pharmacology at Harvard Medical School, thinks that the answer is actually quite simple. "Whenever something slow is happening on the lagging strand, the leading strand simply stops for a while," he says.
The enzyme putting on the brakes for the leading strand is DNA primase, the enzyme that synthesizes the primers for DNA polymerase (Nature 2006, 439, 621). Van Oijen's team used single-molecule techniques to study the kinetics of a multiprotein replication complex in bacteriophage T7, a viral model system requiring only four proteins to reconstitute fully functioning replication machinery.
The single-molecule method involves tethering both ends of the lagging strand, the 5′ end to a glass slide and the 3′ end to a bead. A laminar flow applies a small force that drags the bead and stretches the DNA. Because single-stranded DNA coils up more tightly than double-stranded DNA at the forces used in this experiment, the position of the bead reports the conversion from double-stranded "parental" DNA to single-stranded lagging-strand DNA.
In experiments in which only the leading- strand polymerase is present and thus the single-stranded lagging strand goes unreplicated, van Oijen's team uses effective shortening of the DNA as an indicator of leading-strand synthesis. Plateaus in the length of the leading-strand DNA indicate pauses in its replication. By varying the components included, they determined that primase must be present and functioning for the pausing of the leading-strand synthesis to occur.
This new function of DNA primase appears to be a result of the geometry of the complex, in which six copies of the primase wrap around the DNA. "We think that two of these primases are interacting with each other while they're making the primer. That jams the unwinding activity of the helicase," van Oijen says. "It's like you're biking and somebody throws a stick in your wheel. It just stops."

Van Oijen and his coworkers still need to prove that their model is correct. To do this, they plan to label the individual components of the replication machinery and use additional single-molecule experiments to see how they interact. Using the spectroscopic technique fluorescence resonance energy transfer, or FRET, they will be able to obtain distance information on the nanometer scale.
Van Oijen doesn't think this technique will be directly applicable to more complicated systems. "In a eukaryotic system, replication is taken care of by hundreds of proteins. People have only just begun to understand how this really works," he says.
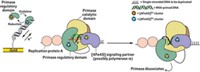
A different puzzle stems from the usual view of leading-strand replication, which implies that it is only primed once. But what happens if replication stops for some reason? One popular model is the "regression model," which proposes that the replication fork backs up, the damage is repaired, and replication picks up where it left off. But this model and others don't account for the observed gaps in the leading strand when replication is blocked.
Professor Kenneth J. Marians and grad student Ryan C. Heller at Memorial Sloan-Kettering Cancer Center in New York City have now studied what happens when DNA replication is blocked by damage on the leading strand. They find that the machinations suggested by the regression model may not be necessary. They instead learn that replication of the leading strand in Escherichia coli can be reprimed and restarted downstream of the damage without the damage first being fixed (Nature 2006, 439, 557). The resulting gap in the leading strand can be filled in later.
So far, they've run experiments modeling the assembly of the machinery after stalled replication forks, but they haven't actually run a replication fork into damage. They don't know what part of the replication machinery can get past the damage or whether all the proteins dissociate and re-form the machinery downstream of the damage. The researchers are currently addressing those issues.
Marians contends that such a system makes particular sense in bacteria, which have only a limited time to replicate their chromosomes. "If you're constantly stopping to repair damage, the odds are that you're not going to be able to replicate your chromosome before cell division starts," he says.
A third puzzle involves the long-known ability of an RNA polymerase to make DNA primers. Primer synthesis for DNA replication typically falls to the primases, enzymes that have evolved expressly for this purpose. Since the early 1970s, however, biologists have known that a virus known as M13 bacteriophage uses RNA polymerase to synthesize primers for DNA synthesis. RNA polymerase is usually involved in the synthesis of RNA during transcription rather than in DNA replication. Because the conformation required for priming is so antithetical to known conformations of RNA polymerase, biologists basically ignored the finding and "put it on the shelf," says Konstantin V. Severinov, a molecular biologist at Rutgers University.
Severinov and his coworkers have now shown that the RNA polymerase in M13 bacteriophage can adopt a previously unrecognized conformation (Nature 2006, 439, 617). In this configuration, the RNA polymerase forms an extended RNA-DNA hybrid bound in a trough that is usually occupied by downstream double-stranded DNA. The 3′ end of the RNA remains available to interact with DNA polymerase, exactly what is needed to prime the DNA polymerase.
Severinov thinks this conformation will turn out to be important for more than just this virus. The M13 bacteriophage has single-stranded rather than double-stranded DNA. "It's probably always going to work this way if transcription of single-stranded DNA takes place," he says.
Severinov speculates that such a complex may also be involved when plasmid DNA (circular DNA found in bacteria) is replicated. "Many pathogenic cells contain plasmids that make them resistant to antibiotics," he says. "It would be nice to get rid of those plasmids. All of a sudden, the cell would become defenseless against the standard arsenal of antibiotics."
At this point, Severinov doesn't know whether the RNA polymerase actively recruits DNA polymerase to the 3′ end of its bound primer. "We would like to know whether communication between transcription and replication occurs," he says.
Each of these three recent studies uses a model system, whether bacterial or viral. "Nobody has reconstituted the eukaryotic replication fork, except in viral systems. There's still precious little known about what drives the eukaryotic replication fork," Marians says.
"It's necessary to take a step back and understand these simple systems before you attempt to understand the mechanistic properties of the complex systems," van Oijen says. "We're trying to increase the complexity bit by bit."
In a Nature commentary accompanying the three papers, Stephen D. Bell of Hutchison MRC Research Center in Cambridge, England, writes: "The power of these reconstituted bacterial and bacteriophage systems is apparent in the exquisite mechanistic detail that can be gleaned from their analysis. Similarly detailed studies of the more complex primases found in higher organisms are eagerly anticipated."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter