Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
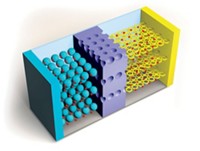
Materials
Lithium Batteries With More Muscle
Innovations in battery materials are leading to applications heftier than portable electronics
by Mitch Jacoby
June 12, 2006
| A version of this story appeared in
Volume 84, Issue 24

Think of lithium-ion batteries and the first things likely to come to mind are cell phones, laptop computers, and other portable electronic gadgets. No doubt about it, the low weight, small size, and large energy capacity of lithium-ion batteries relative to other battery types have helped these high-tech power packs corner the market in the portable electronics arena. But that's not all they're good for.
Lithium-ion batteries are now muscling their way into all sorts of applications that seemed implausible just a few years ago. These days, the batteries are being used to energize powerful electric drive systems in automobiles and motorcycles. They're also being tapped for medical and backup power service and other applications that demand a big jolt of power.
"Lithium-ion batteries are moving into applications where they were never used before," says Ric Fulop, founder of A123 Systems, a new battery company based in Watertown, Mass. A123 manufactures high-powered lithium-ion batteries for a number of applications, including a new line of 36-V DeWalt power tools (C&EN, Feb. 13, page 79). The tools, which just hit store shelves a few weeks ago, were on display last month at the Advanced Automotive Batteries conference in Baltimore. The meeting focused on advances in hybrid-electric-vehicle batteries and related topics and featured presentations on numerous subjects, including the evolution of cathode, anode, and electrolyte materials that have played key roles in the changes in lithium-ion battery usage.
According to M. Stanley Whittingham, a chemistry professor at the State University of New York, Binghamton, changes in lithium-ion batteries over the years have been largely incremental. Widely recognized as the inventor of lithium-ion batteries, Whittingham headed the Exxon research team that developed the first commercial rechargeable lithium battery in 1977. That battery featured a titanium sulfide (TiS2) cathode and a lithium-aluminum (LiAl) anode.

Whittingham notes that although the titanium sulfide battery was technologically sound, it wasn't a commercial success. Success with lithium-ion batteries came some 15 years later when Sony introduced a battery that incorporated a lithium cobalt oxide (LiCoO2) cathode and a lithium-intercalated carbon (LiC6) anode. The cobalt-based battery was a hit with portable electronics applications because of its ability to store a lot of energy in a small, lightweight package.
More recently, manufacturers began making batteries with cathodes that include transition metals such as nickel and manganese, in addition to cobalt. The stoichiometry and other properties of the mixed metal oxide cathodes have been tuned to enhance charge capacity, power output, recharge time, and other performance parameters, thereby customizing the batteries for all sorts of new applications.
Regardless of electrode composition, lithium-ion batteries undergo similar electrochemical reactions. As the battery is charged, lithium ions migrate from the cathode and insert themselves (intercalate) in the anode. Charge-balancing electrons also move to the anode but travel through an external circuit in the charger. On discharge—meaning when the battery is used to provide power—the reverse process occurs, and electrons flow through the device being energized.
Structurally, the older and newer cathode materials are similar in that they consist of layers of metal oxide octahedra or metal sulfide octahedra between which reside layers of lithium ions. As Whittingham notes, the purity of the layers and the orderliness with which they stack govern a large number of electrochemical properties and therefore have been the focus of intense research.
In the as-synthesized state, layered electrode materials tend to stack neatly. But upon repeated charging and discharging, the alternating layers undergo lateral shifts with respect to one another. Whittingham explains that the structural disorder reduces the electrodes' ability to conduct lithium ions and ultimately leads to a loss of a battery's charge capacity.
A common way to minimize the disorder in electrode materials and associated loss of charge capacity is to design the electrochemical cell such that only about half of the lithium ions cycle back and forth between the anode and cathode. Running the reaction to completion will destroy the electrodes, Whittingham points out.
In the case of Li(NiMnCo)O2-type cathodes, a key factor in electrochemical performance is the location of the transition-metal ions, which can migrate from their layer into the lithium layer. As it turned out, through a combination of X-ray- and neutron-diffraction studies, Whittingham's group and other research groups determined that cobalt and manganese stay put−only nickel moves to the lithium sites. Furthermore, the researchers found that the extent of nickel's migration can be controlled or stopped altogether by tailoring the concentration of cobalt and by reducing the synthesis temperature below conventional temperatures (approximately 900 °C).
But forcing all of the nickel ions into the transition-metal layer may not be such a good idea after all. "Nickel in the lithium layer performs a critical role," Whittingham stresses. It pins the layers together, he says, and thereby stops the relative shifting between adjacent layers that leads to loss of charge capacity.
Numerous other strategies have been used to improve properties of electrode materials. At the Naval Research Laboratory in Washington, D.C., for example, Arnold M. Stux and Karen E. Swider-Lyons blend LiCoO2 with good electronic and lithium-ion conductors in an effort to lower battery resistance and improve overall cell performance. In a recent study, the team found that blends of LiCoO2 and Li2RuO3 outperformed either of the pure materials in terms of charge capacity and operating voltage, especially at high charge and discharge rates (J. Electrochem. Soc. 2005, 152, A2009).

On the basis of electrical measurements, the researchers explain that the blends are effective because the components do not interact strongly, as they would, for example, if one material coated the other. As such, the materials function in parallel (not in series). That arrangement decreases cell resistance and increases charge capacity.
One material that's causing quite a stir in the battery world nowadays is lithium iron phosphate (LiFePO4), the relatively inexpensive and environmentally friendly material found in the cathodes of A123's new high-powered batteries. According to A123 research scientist Andy Chu, the company's batteries are an outgrowth of breakthrough work by Massachusetts Institute of Technology materials science professor Yet-Ming Chiang. Nearly four years ago, Chiang discovered that doping the ordinarily weak conductor LiFePO4 with zirconium, niobium, and other cations leads to an amazing eight-order-of-magnitude increase in conductivity (Nat. Mater. 2002, 2, 123).

Although the performance of the new lithium iron phosphate batteries may impress industry observers and construction workers, some researchers question the basis of the enhanced conductivity. At the University of Waterloo, chemistry professor Linda F. Nazar and coworkers reproduced the MIT work and conductivity results. The group concluded that, contrary to the doping-based explanation offered by Chiang, the enhanced conductivity results from other factors, as they were able to achieve high conductivities without doping. The Waterloo team explains that doping tiny electrode particles with zirconium alkoxide and other metal alkoxides leaves behind excess carbon that reacts with LiFePO4 and forms metal phosphides that can be detected readily. According to Nazar, it's the metal-rich phosphide phase, which was observed by the Waterloo group and others, that's responsible for the enhanced conductivity (Nat. Mater. 2004, 3, 147).
While some researchers focus on novel cathode materials, others study replacements for anodes and other battery components. Martin Winter, a professor at the Institute for Chemistry at Graz University of Technology, in Austria, says a variety of materials are being studied as replacements for the common carbon anode used in many of today's lithium-ion batteries. For example, lithium-storage metals, including tin, silicon, and aluminum, have much higher charge densities than graphitic carbon and can form reversible lithiated phases such as Li22Si5 and Li22Sn5. But those materials are subject to enormous volume changes upon lithiation that can lead to mechanical failure.
In addition, partially fluorinated liquid electrolytes are under investigation as substitutes for ethylene carbonate, dimethyl carbonate, and other currently used electrolytes. Winter points out that the fluorinated substitutes offer improved performance at low temperature, oxidation resistance, and reduced flammability.
Advances in materials properties have helped make lithium-ion batteries the power sources of choice for a range of new applications. But as A123's Chu points out, the electrode materials don't act alone. They are brought together with new electrolyte systems and new cell designs and coupled with advances in engineering and manufacturing to produce today's lightweight yet powerful lithium-ion batteries.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter