Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Peptides Trapped On A Thread
Synthetic peptide rotaxanes may provide insights into natural interlocked systems
by Michael Freemantle
January 25, 2006

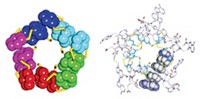
Rotaxanes derived from synthetic peptide macrocycles and nonpeptidic diammonium threads have been prepared by chemists at the University of Edinburgh, Scotland (J. Am. Chem. Soc., published online Jan. 19, dx.doi.org/10.1021/ja057206q).
A rotaxane is a bead-on-a-string type structure in which a linear molecule threads through at least one cyclic molecule. Bulky components at each end of the linear molecule stop the assembly from unraveling.
“We’ve prepared the first rotaxanes that utilize cyclic peptides as the macrocyclic component,” says professor of organic chemistry David A. Leigh, who led the synthesis team.
“The work is significant,” he adds, “because rotaxane architectures have been discovered in natural peptides in recent years.” One notable example is the antibacterial 21-amino-acid peptide microcin J25 (C&EN, Nov. 10, 2003, page 46). “Such mechanically interlocked peptides display a wealth of intriguing properties such as high resistance to peptidases, unique modes of antimicrobial and antiviral action, impressive membrane transport characteristics, and stability to thermal and chemical denaturing,” Leigh says.
His group synthesized the rotaxanes from a class of well-known synthetic cyclic peptides consisting of four or five repeating l-prolylglycyl units. The threads, with bulky stoppers at each end, were prepared from cationic diols containing ethane-1,2-diammonium or butane-1,4-diammonium moieties that act as templates for the cyclic components.
The rotaxanes assemble by hydrogen-bonding between the amide groups of the peptides and the diammonium thread units. “The hydrogen bonding is able to disrupt the intramolecular amide-amide hydrogen bonding in the cyclopeptides to such an extent that threading to form a rotaxane architecture becomes thermodynamically favorable,” Leigh explains. “Even though the rotaxane yields are similar (56–63%), the ethyldiammonium template seems to hold the cyclic peptides strongly in place, whereas the butyldiammonium binding site is somewhat weaker.”
The Edinburgh researchers are now trying to extend their methodology to make rotaxanes in which both the cyclic and thread components consist of peptides. “Ultimately, we would like to have generic methods for interlocking peptides that could be used to make microcin J25 and other naturally occurring interlocked peptides,” Leigh says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter