Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Pruning Blood's Sugars
April 2, 2007
| A version of this story appeared in
Volume 85, Issue 14
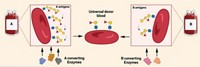
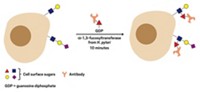
Blood transfusions can go terribly awry if our immune systems detect red blood cells that possess foreign surface sugars: type A's α-1,3-linked N-acetylgalactosamine or type B's α-1,3-linked galactose. Now, clipping off a red blood cell's type A and type B sugars to make the more universally accepted O-type blood cell has been achieved with two newly discovered bacterial glycosidases (Nat. Biotechnol., DOI: 10.1038/nbt1298). Previous strategies to trim these potentially immunogenic sugars from A-, B-, and AB-type blood have not found clinical use because of enzymatic inefficiency. The glycosidases identified by a team led by Henrik B. Clausen at the University of Copenhagen do the conversion with excellent yield at neutral pH. The authors point out that "the availability of these bacterial enzymes should allow efficient and cost-effective enzymatic conversion of blood group A, B, and AB red blood cells to universal red blood cells." Furthermore, one such enzyme, α-N-acetylgalactosaminidase, has both an unusual catalytic mechanism and a novel glycosidase structure.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter