Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Lanthanide Spectral Arsenal
May 21, 2007
| A version of this story appeared in
Volume 85, Issue 21
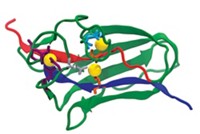
Lanthanide metal ions are gaining in popularity as a means to increase the power of spectroscopy for studying proteins. In the latest example, Karen N. Allen of Boston University, Barbara Imperiali of MIT, and Harald Schwalbe of Johann Wolfgang Goethe University, in Frankfurt, Germany, teamed up to create peptide-based tags that bind two lanthanide ions and can be added easily to proteins (J. Am. Chem. Soc., DOI: 10.1021/ja070480v). Attached to the protein ubiquitin and loaded with Tb3+, the tags provided strong luminescence signals for protein detection. When loaded with the paramagnetic lanthanides Tm3+ and Eu3+, the tags helped to orient ubiquitin proteins for NMR spectroscopy structure determination. In a second paper, the researchers used tags with Tb3+ (shown, silver balls = Tb3+) to determine the phases of diffracted X-rays to solve ubiquitin's crystal structure (J. Am. Chem. Soc., DOI: 10.1021/ja070481n). The tags could become valuable tools, the researchers say, especially for studying eukaryotic proteins for which existing approaches, such as the incorporation of selenium for X-ray phasing, have proven difficult.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter