Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Dispute Over Crystal Structure Nomenclature Takes Center Stage
by Ann M. Thayer
June 18, 2007
| A version of this story appeared in
Volume 85, Issue 25
At a recent international conference on crystallization, participants spent some time discussing the chemical definitions of and appropriate terminology for multicomponent crystals. "It was probably one of the low points of my career," one attendee tells C&EN, "when we spent 30 minutes arguing whether the word should be spelled co-crystal or cocrystal."
COVER STORY
Dispute Over Crystal Structure Nomenclature Takes Center Stage
To those working in the crystal engineering field, this probably isn't surprising because there's been a vigorous debate going on. Although different terminology for solid forms had been cropping up for a long time, the controversy took off in 2003 in the Royal Society of Chemistry journal CrystEngComm and then continued more recently in the American Chemical Society's Crystal Growth & Design .
Arguments center most heatedly around the definitions and uses of the terms cocrystal and pseudopolymorph. One reason people care is that the field has taken on a new purpose as higher stakes players have gotten involved. Pharmaceutical companies are increasingly keen about finding new solid forms of their drug compounds and need terminology by which to refer to them in patents or classify them in regulatory filings.
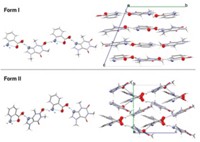
Crystals containing two or more components—historically called, among other things, molecular complexes, inclusion compounds, channel compounds, and clathrates—have been known and studied for more than 100 years. Entrenched terms include salts, hydrates, and solvates, in which one of the components is clearly an ion, water, or solvent molecule, respectively. Crystals containing an active pharmaceutical ingredient as the "host" and something other than a solvent or water as the "guest" are increasingly being referred to as cocrystals.
G. Patrick Stahly, former chief operating officer of Aptuit's solid-state chemistry business SSCI, gives an overview of the debate and suggestions for dealing with terminology in a recent review article (Cryst. Growth Des. 2007, 7, 1007). He says both crystalline salts and cocrystals are multicomponent solids. A crystal in which a proton is clearly transferred between an acid and base is a salt, whereas all other multicomponent forms can be considered cocrystals.
In this classification scheme, for example, solvates and hydrates are types of cocrystals. This terminology, Stahly suggests, is descriptive, understandable, and worth keeping. He questions, however, the need or utility of making any further distinction on the basis of the properties of a guest or method of making a cocrystal.
The late Margaret C. Etter of the University of Minnesota helped popularize the term cocrystal about 20 years ago. Others have since used it to describe only those multicomponent crystals in which the guest itself is a solid at room temperature; Kansas State University chemistry professor Christer B. Aakeröy is often credited with making this distinction (CrystEngComm 2005, 7, 439).
Arguments against the term cocrystal and in favor of molecular complex were made in 2003 by University of Hyderabad chemistry professor Gautam R. Desiraju and disputed by emeritus chemistry professor Jack D. Dunitz at the Swiss Federal Institute of Technology, Zurich (CrystEngComm 2003, 5, 466 and 506).
But what probably stirred up even more persistent controversy was Desiraju's support of the term pseudopolymorph. According to Desiraju and others, the late Walter C. McCrone coined the term in the mid-1960s. Even as its defender, Desiraju admits that definitions in the literature are confusing (Cryst. Growth Des. 2004, 4, 1089).
The general thinking has been to use the term to describe host-guest complexes, usually solvates and hydrates. By differing compositionally and structurally from polymorphs (or crystalline forms) of the host, they are therefore pseudopolymorphs. Desiraju has even suggested quasipolymorph, and others have put forth elaborate terms such as solvatomorph, hydratomorph, and pseudopolymorphic solvate.
Desiraju's support of the term pseudopolymorph led Kenneth R. Seddon, a chemistry professor at Queen's University, Belfast, to opine against it as "unnecessary and misleading jargon" (Cryst. Growth Des. 2004, 4, 1087). Likewise, chemistry professor Joel Bernstein of Ben-Gurion University, in Israel, has expressed his lack of enthusiasm (Cryst. Growth Des. 2004, 4, 1661). They seem to share the view that there's nothing "pseudo" about solvates and hydrates, which are distinct enough to have separate descriptors.
Seddon went so far as to recommend that pseudopolymorph be banned from appearing in the literature. Taking exactly the opposite view, Hyderabad chemistry professor Ashwini Nangia defended the term. His principal arguments were that it and related terms were already widely used and were "more than mere scientific terms," having "entered the legal lexicon" (Cryst. Growth Des. 2006, 6, 2).
It's unclear what, if any, practical difference all this makes and what bearing it has on new solid forms. "Whether you call it a cocrystal, a hydrate, a solvate, or a cabbage doesn't really matter to me," one solid-state chemist tells C&EN. "At the end of the day, what we care about is improving the physical properties and turning something that's like brick dust into a billion-dollar drug product."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter