Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
FDA Proposes Sunscreen Rating System
September 3, 2007
| A version of this story appeared in
Volume 85, Issue 36
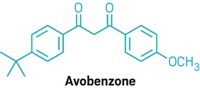
FDA has proposed a long-awaited UV-A sunscreen effectiveness rating system and testing protocol. Sunscreen ingredient makers, formulators, and consumers have waited eight years for the agency to provide guidance on how effectively creams and lotions protect against UV-A rays, which scientists believe cause skin-wrinkling and contribute to skin cancer. The rating system would assign one to four stars to a product based on new in vitro and in vivo tests, with one star corresponding to the lowest level of protection and four stars indicating the highest. The star rating system is similar to one developed 15 years ago in the U.K. by Boots Co. Ciba Specialty Chemicals, a leading supplier of sunscreen active ingredients, says the proposed system is a "clear step in the right direction." For 30 years, U.S. consumers have relied on sun protection factor (SPF) ratings, which only indicate the ability of a sunscreen to block the UV-B rays responsible for sunburn. The confusion over how effectively sunscreens protect people from the harmful effects of the sun led to the filing of a consumer fraud lawsuit last year in California against a number of sunscreen manufacturers. FDA's proposal would also allow new combinations of avobenzone, a UV-A filter, and other sunscreen active ingredients.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter