Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Model Devised for Polyolefin Catalysts
September 24, 2007
| A version of this story appeared in
Volume 85, Issue 39
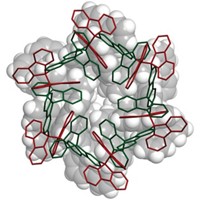
Producing polyolefins via Ziegler-Natta catalysts is one of the most fundamental industrial chemical processes. Piotr Sobota of the University of Wrocław, in Poland, and coworkers now have devised a model system that for the first time provides a clear picture of the active-site structure of an important commercial polyolefin catalyst (Inorg. Chem., DOI: 10.1021/ic7013094). Polyolefin catalysts typically are formed from a precursor containing magnesium and titanium chlorides or alkoxides. For the catalyst studied, the precursor is Mg3Ti(OR)8X2 (R = alkyl, X = o-cresol or OCH2CH3). But the exact structure of the active catalyst is unknown. The researchers instead created an isolable manganese mimic containing a Mn3Ti unit and obtained its crystal structure. This model permitted them to visualize the polymerization process. They suggest that the metal atoms of the catalytic site, in which the titanium atom occupies a chiral position, impose a chiral orientation to the growing polymer chain. This effect guides the head-to-tail insertion of olefin units into the chain, which results in a stereoregular polymer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter