Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Zinc Binding Controls Protein Interactions
October 22, 2007
| A version of this story appeared in
Volume 85, Issue 43
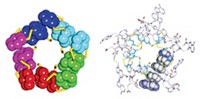
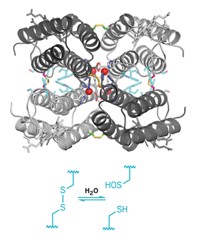
Protein-protein interactions, which are key to nearly all cellular processes, are guided by formation of many weak, noncovalent bonds (such as hydrogen bonds) spread over large molecular surfaces. Inspired by the use of metal ions and small organic ligands to build supramolecular complexes, F. Akif Tezcan, Eric N. Salgado, and Jasmin Faraone-Mennella of the University of California, San Diego, now show that the strength and selectivity of a few metal-ligand bonds alone can control the assembly of multiprotein structures (J. Am. Chem. Soc., DOI: 10.1021/ja075261o). The researchers demonstrated the concept by adding Zn(II) to cytochrome cb562, a four-helix bundle heme protein. They first modified the protein to contain pairs of histidine residues on one helix that can bind zinc ions, which subsequently nucleate protein-protein interactions. Altogether, four zinc atoms and four protein molecules self-assemble into 16-helix macromolecules. These aggregates are readily dissolved by adding a metal chelator, such as EDTA, or by lowering the pH below 6. The researchers believe metal-ion control of protein-protein interactions could lead to new biomaterials or manipulation of cellular processes.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter