Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Biotech Challenges
Earnings drop in third quarter at both Amgen and Biogen but increase 22% at Genentech
by Lisa M. Jarvis
November 19, 2007
| A version of this story appeared in
Volume 85, Issue 47
With portfolios aging, big biopharmaceutical companies are having a difficult time reaching the double-digit sales and profit growth that financial analysts have come to expect. A dearth of new products and challenges to older ones have made this difficulty particularly acute in the third quarter.
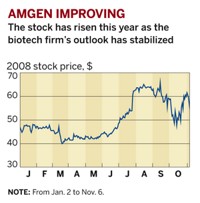
Among the major firms, Amgen is facing the most pressure this year after questions arose about the safety of its portfolio of erythropoietin-stimulating agents (ESAs), which are used to treat anemia in cancer and kidney dialysis patients. The situation has taken its toll on the company's bottom line; Amgen's income dropped 3.5% to $1.2 billion in the third quarter from the 2006 quarter, while revenues were flat at $3.6 billion.
Quarterly sales of the ESA Aranesp fell 23% to $818 million, a decline the company attributed to weak U.S. demand after the Centers for Medicare & Medicaid Services (CMS) decided to limit its reimbursement of the drug. Amgen said earlier this month that it would submit new information to CMS in hopes that the agency will reconsider its decision.
A combination of lower dosing by doctors and bigger price discounts by the company took sales of Epogen, Amgen's other ESA, down 5% in the quarter to $602 million. Yet the company also won a court battle with Roche, which has been trying to bring its own erythropoietin product, Cera, to the U.S. market. A federal district court in Boston ruled that the drug, which would have threatened sales of Epogen, violates Amgen's patent.
Products aside from the ESAs were steady "but not humming," says Morgan Stanley analyst Steven Harr. Combined sales of Neulasta and Neupogen, which treat a blood cell disorder in cancer patients, were up 10% to $1.1 billion. Enbrel, for arthritis and psoriasis, had sales of $821 million, a 16% increase.
Amgen appears to have little chance of refreshing its fading portfolio anytime soon. Sales of the company's newest drug, Vectibix, which is used to treat colorectal cancer, have been on a steady decline after the release of an unfavorable study earlier this year. Third-quarter sales totaled $41 million, compared with $45 million in the second quarter and $51 million in the first.
And though most analysts have high expectations for denosumab, an osteoporosis treatment that could reach the market by 2009, it may not be the multi-billion-dollar drug Amgen has been counting on. From conversations with doctors who treat a high volume of osteoporosis patients, James Reddoch, an analyst at Friedman, Billings, Ramsey Group, predicts denosumab will likely be used as a second-line agent and reap sales of about $800 million in its peak year.
Meanwhile, Biogen Idec, which has had an even tougher time getting out of the old-product rut, had another lackluster quarter. Though revenues increased 12.2% in the third quarter 2007 to $789 million, profits plummeted 17.7% to $170 million.
Biogen Idec missed analysts' sales estimates for virtually every drug in its portfolio. The multiple sclerosis drug Tysabri brought in $63 million in the quarter, $4 million to $6 million less than what Cowen & Co. and Morgan Stanley had expected. At $455 million, third-quarter sales of Avonex, its other multiple sclerosis drug, were approximately 5% below estimates and 2% below the prior year's sales.
Only Biogen Idec's share of sales from Genentech's Rituxan, used to treat non-Hodgkin's lymphoma and rheumatoid arthritis, met forecasts of $235 million. Yet under the agreement between the two companies, Biogen Idec stands to lose its share of those royalties in 2009.
The company's third-quarter performance did little to inspire confidence in its ability to attract suitors. Last month, Biogen Idec put itself up for sale, stating that shareholders would gain if the company was combined with another entity.
Big pharmaceutical companies are keen to forge deeper into the biotech world, and Biogen Idec would provide key biopharmaceutical manufacturing plants and a reasonably solid pipeline of new drugs. Yet with sales of its current products somewhat weak, analysts question whether anyone will lay out the cash—more than $20 billion—Biogen Idec seems to expect for itself.
The company's third-quarter performance did not "change our view that while Biogen Idec has a number of intriguing strategic assets, the current price is likely too expensive for a sale given long-term growth concerns," Morgan Stanley's Harr says.
Genentech looks to be the strongest among the big biotechs, but it is also heavily reliant on the colon and lung cancer drug Avastin. The company enjoyed a 22.1% rise in third-quarter profits to $778 million and a 19.6% jump in sales to $2.3 billion.
Sales of Avastin were up 37% to $597 million, and Genentech's future appears tied to the drug maintaining that robust level of growth. The company is awaiting Food & Drug Administration approval to use Avastin as the first line of attack in patients with metastatic breast cancer. Analysts agree the drug's greatest potential is as an add-on in patients whose cancer has spread. "Adjuvant cancers are key to the company's four- to seven-year growth rate," Harr notes.
Meanwhile, sales of Genentech's other core products were steady in the quarter. The breast cancer drug Herceptin brought in $320 million, a 6% increase, while the allergy drug Xolair had sales of $121 million, up 13%. Sales of Rituxan totaled $572 million, a 12% improvement.
Genentech is also struggling with competition from one of its own products. Ophthalmologists have been using Avastin rather than Lucentis, a newly launched—and expensive—fragment of the Avastin antibody, to treat wet age-related macular degeneration, a serious eye disease.
To combat the practice, Genentech announced in October that it would stop shipping Avastin to compounding pharmacies, which had been diluting the drug to treat the disease. After an outcry from physician advocacy groups, Genentech agreed to temporarily continue sales while it evaluates the situation.
Meanwhile, the National Institutes of Health is initiating a study comparing the efficacy of Lucentis and Avastin; the first results are expected in 2009.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter