Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Really Strong Triple H-Bonding
Maximizing the number of secondary electrostatic interactions in acceptor-donor complexes boosts binding constants
by Bethany Halford
January 4, 2007
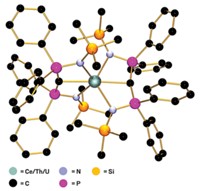
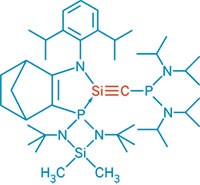
The strongest triple-hydrogen-bonded complex measured to date has been reported by chemists in Europe (J. Am. Chem. Soc., DOI: 10.1021/ja067410t).
A group led by David A. Leigh and Hamish McNab of Scotland's University of Edinburgh and Francesco Zerbetto of Italy's University of Bologna designed the complex shown with all three H-bond acceptors (A) in one molecule and all three H-bond donors (D) in another. Systems with this AAA-DDD motif are known to associate far more strongly than complexes with an ADA-DAD pattern because they provide the maximum number of attractive secondary interactions between the H-bonding partners. By tweaking the aromatic framework of a known AAA molecule, the team was able to create an H-bond network that's more than 100 times stronger than what's been observed previously.
"Our results show the extraordinary significance of these secondary interactions," Leigh says. "They can make the difference between a triple hydrogen-bonded complex binding constant being 90 M-1 and being 20 million M-1."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter