Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Brute Force Breaks Bonds
Ultrasound directs ring-opening reactions in more than expected ways
by Rachel Petkewich
March 22, 2007
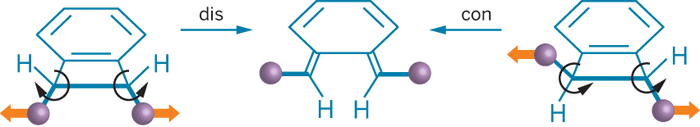
Chemists commonly use heat, light, pressure, or electricity to kick-start reactions. Taking a different tack, Jeffrey S. Moore, Charles R. Hickenboth, and colleagues at the University of Illinois, Urbana-Champaign, have used mechanical energy to trigger ring openings and, in the process, steer the reactions away from products predicted by orbital symmetry rules (Nature 2007, 446, 423).
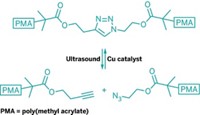
In this case, the mechanical energy is provided by the collapse of cavitation bubbles that form in solution during exposure to ultrasound. Applying ultrasound to accelerate reactions is not new, but it has never caught on for organic synthesis because it has tended to generate a mix of products. Ultrasound has been used to fracture large molecules, such as polymers, in nonspecific locations and thus create a variety of carbon radicals.
The new work is precise, says Martin Beyer, a research chemist at Technical University of Berlin. "For the first time, the effect of ultrasound is unambiguously related to mechanochemical activation of a specific site." He adds that this advance shows the potential of mechanical force in organic synthesis, especially for natural products and medicinal chemistry, and that "it may very well be that nature uses mechanochemistry for enzymatic synthesis of complex compounds."
Directing force into small molecules is difficult. Therefore, the Illinois researchers linked mechanically sensitive 1,2-disubstituted benzocyclobutene isomers to two polyethylene glycol chains, applied ultrasound, and analyzed the bulk solutions by using 13C nuclear magnetic resonance spectroscopy. The spectra revealed that both the cis and trans isomers provide the same ring-opened product, the one expected from the trans isomer.
The Woodward-Hoffmann rules of orbital symmetry predict that for these ring-opening reactions each isomer of the reactant will form a different isomer of the product. So what makes this situation different?
Moore explains that thermal energy subjects reactants to random Brownian motion, whereas mechanical energy provides a direction to atomic motions. Therefore, forces from ultrasound efficiently direct the energy by straining the molecule, and thus reshaping the potential energy surface, he says.
The claims are bold, but they bear out, according to a Nature commentary by chemistry professor Virgil Percec and graduate student Brad M. Rosen at the University of Pennsylvania. "Although chemists might not immediately rush to adopt this technique, the work is a remarkable first step in using mechanical stress to break bonds in a synthetically useful organic reaction," they write.
Because the molecular rearrangement occurs before the polymer chain breaks, Moore notes the possibility and benefits of incorporating mechanically sensitive compounds into materials like climbing or parachute ropes. The researchers hope to link the mechanical component to a visual indicator so, for example, the stress in a rope could be assessed before one uses the rope to rappel down a mountain.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter