Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cleaved Ether Shines To ID Trace Palladium
Metal gives itself away by snipping a C–O bond in a sensor molecule, causing the sensor to fluoresce
by Bethany Halford
September 27, 2007

Palladium reagents are prized for their powerful ability to catalyze covalent bond formation. But that usefulness has a downside: Residual palladium is notoriously difficult to remove from final products and from chemical reactors. Furthermore, expensive analytical spectrometers are usually needed to detect trace amounts of the metal.
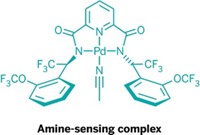
To help work around this problem, University of Pittsburgh chemists have now developed a highly sensitive fluorescent sensor for detecting palladium at levels as low as 1 ppm (J. Am. Chem. Soc., DOI: 10.1021/ja073910q).
The fluorescein-based sensor, developed by Kazunori Koide, Fengling Song, and Amanda L. Garner, capitalizes on palladium's ability to catalytically cleave the C???O bond of allylic ethers. In the presence of Pd(0) or Pd(II) under reducing conditions, the allylic ether in the sensor molecule is cleaved. The resulting alkoxide fragment fluoresces, indicating the presence of palladium.
The researchers think the user-friendly sensor will be helpful for monitoring the presence of palladium in pharmaceuticals and in chemical reactors as well as for detecting palladium in mining operations.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter