Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Switches For The Body Electric
Double-bond isomerization makes new ways to control nerve impulses available
by Carmen Drahl
March 24, 2008
| A version of this story appeared in
Volume 86, Issue 12
ONE LITTLE BOND—the double bond—is beginning to make a big difference in neuroscience.
Under the right chemical circumstances, double-bonded compounds can respond to light by converting between trans and cis isomers. Research teams can now harness these chemical shapeshifters to generate nerve impulses in worms, fish, and rats at will, with the help of a beam of light. Although the technology is in its infancy, the groundwork could lead to a better understanding of, and new treatments for, conditions such as spinal cord injury, Parkinson's disease, and vision loss.
Normally, nerve impulses are produced by voltage-gated ion channels, pores that sense changes in local charge distribution across nerve cell membranes. They open and close, ushering ions into and out of cells to propagate electrical signals. But the new light-based tools create torrents of ions in other ways. Some researchers shuttle ions by means of natural proteins gated by light-activated double-bond latches. Meanwhile, one team is modifying naturally occurring ion channels with synthetic latches.
Exploiting light to control the nervous system isn't a new idea. For two decades, researchers have attempted to control nerve signaling with light-activated neurotransmitters, which are small molecules that carry messages across gaps between neurons. By "caging" small molecules such as glutamate with a light-activated functional group, the neurotransmitters become inactive unless set free by a pulse of light. Unfortunately, it is difficult to target caged compounds to a particular part of the nervous system. "We can't do experiments with these compounds in live animals," says Case Western Reserve University neuroscientist Stefan Herlitze.
Using isomerization-based techniques, scientists can now target specific types of neurons. Until these tools arrived on the scene, "we never had a good way to tell a group of neurons what to do," says Timothy A. Ryan of Weill Medical College at Cornell University, a biophysicist who studies synapses, the specialized junctions through which nerve cells signal each other.
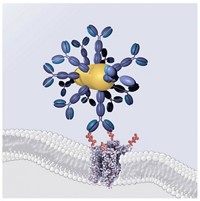
One component of this powerful technology had its beginnings in an unusual place—pond scum. Single-celled algae in ponds orient themselves to light by using a light-sensitive membrane-spanning protein called channelrhodopsin-2. This channel's responsiveness to light comes from retinal, a vitamin A-derived cofactor that's bound to the inside of the protein pore. In response to blue light, a key double bond in retinal isomerizes from trans to cis; the change allows positively charged ions, including sodium ions (Na+), to flow through the pore and into the algae cells. "The basic idea is that these proteins convert a photon of light into a current," Herlitze says.
That response is similar to what happens when a nerve cell detects a signal. The signal causes voltage-gated Na+ channels in the nerve cell membrane to open and allow sodium ions to rush into nerve cells, making the cell interior more positively charged.
In 2005, three independent teams of researchers, including Herlitze's group, capitalized on this similarity. They expressed the gene for channelrhodopsin in neurons. Shining blue light on the nerve cells made the light-gated channels do the voltage-gated channels' job of turning on electrical signals.
Next, researchers sought a way to silence nerves in response to a different wavelength of light. Last year, they found their "off switch" in halorhodopsin, a membrane protein found in microbes that grow in extremely salty habitats. Halorhodopsin carries chloride anions (Cl-) to the inside of the cell in response to yellow light. The anions adjust the positive charge inside the cell and turn off the flow of electrical signals. Although halorhodopsin responds to a different wavelength of light, its pore, like channelrhodopsin's, has a retinal gate.
DIFFERENT AMINO ACIDS surround retinal in each protein, and this probably accounts for the differences in light sensitivity, according to Alexander Gottschalk, a neuroscientist from Johann Wolfgang Goethe University in Frankfurt who helped develop the halorhodopsin technology. Dieter Oesterhelt at the Max Planck Institute of Biochemistry in Martinsreid, Germany, solved halorhodopsin's X-ray crystal structure in 2000, but there have been no channelrhodopsin structures solved yet.
Gottschalk and his collaborators tested the "on" and "off" switches by expressing genes for channelrhodopsin and halorhodopsin in nerve cells. To turn on the electrical signal, they used blue light to activate channelrhodopsin, causing a large influx of Na+. To turn it off, they used yellow light to activate halorhodopsin, causing an influx of Cl-, which counteracts the charge from the Na+ influx. This buffering effect differs from what happens naturally in nerve cells, but it appeared to achieve the same biological results.
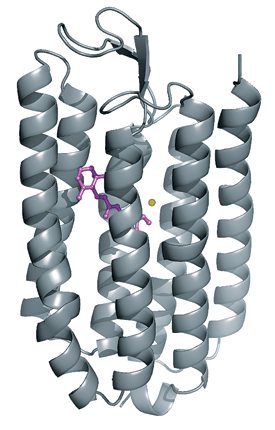
Halorhodopsin is a membrane-spanning protein that pumps Cl- (yellow) into cells. Yellow light isomerizes a double bond in a retinal cofactor (purple), letting Cl- pass.
WITH BOTH "on" and "off" switches for nerve cells in hand, the team tested them in a live organism. They expressed genes for both types of switches in a worm, targeting a specific type of neuron involved in motion. Blue light made the worm contract its muscles, and yellow light made it relax them.
At last month's Biophysical Society Annual Meeting in Long Beach, Calif., Gottschalk presented preliminary work targeting light-gated channels to additional types of neurons in worms, including dopamine-producing neurons.
Dopamine-producing neurons cause worms to slow their pace when they encounter food. Gottschalk used channelrhodopsin to stimulate these neurons at will, making worms slow down on command, as if food were around.
These worms, Gottschalk said, may someday be useful in screens for drugs to combat Parkinson's disease. Parkinson's is thought to be caused by deterioration of dopamine-producing neurons. Killing or damaging dopamine-producing neurons to mimic Parkinson's in the souped-up worms would hamper their response to flashes of light. Researchers could treat damaged worms with drug candidates and then look for worms that recover their reaction to light to find hits. However, more work is necessary to realize that goal, Gottschalk said.
At the meeting, Herlitze presented preliminary work that illustrates another potential use of light-gated channel proteins in medicine. He paralyzed the left side of a rat's diaphragm, an organ crucial for breathing. The rat's breathing became shallower, as though it had experienced a spinal cord injury. Then he injected a virus encoding channelrhodopsin into motor neurons on the defective side of the diaphragm. With a flash of blue light, he made the defective side of the diaphragm contract again, improving the rat's breathing.
Herlitze also described another light-gated tool: a visual protein from rats. Rhodopsin is a light-sensitive membrane-spanning receptor in the eyes of vertebrates such as rats and humans that helps in adaptation to dim light. Like channelrhodopsin and halorhodopsin, rhodopsin is also gated by one of retinal's double bonds. But in this case, isomerization doesn't lead directly to a rush of ions.
Rhodopsin is a G-protein-coupled receptor, one of the most important families of proteins in the human body. It opens ion channels indirectly via a G-protein messenger. Rhodopsin's bound retinal starts out in the cis form. Light converts retinal to its trans isomer and causes a conformational change in rhodopsin that activates the G protein. The G protein then dissociates from rhodopsin to relay its message to ion channels. Herlitze has genetically transplanted this system into neurons, and he has used it to control nerve impulses in a developing chick's spinal cord.
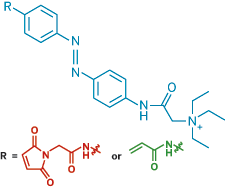
In a light-activated switch, replacing a cysteine-reacting group (red) with an acrylamide (green), which reacts with a range of electron-rich amino acids, lets researchers make light-controlled K+ channels that do not have to be genetically engineered to contain cysteine.
This system, although more indirect than channelrhodopsin or halorhodopsin, holds great promise, according to Herlitze. The G-protein middleman can, in principle, be changed so that light could be used to control signals to other proteins, not just ion channels. Since G-protein-signaling pathways are implicated in diseases ranging from ulcers and migraines to cancer and stroke, the potential, he told C&EN, is enormous. "Vertebrate rhodopsin is a great tool, and not that many people have jumped on it yet," he said.
Retinal-gated proteins have given researchers unprecedented control over nerve impulses, and many groups are using the technology. However, these tools may not be ideal for every application. They require delivering foreign genes to nerves, either during development or, as Herlitze did with the breathing-impaired rat, with a virus. "There are problems with that delivery system," such as the risk of provoking an immune response, says neuroscientist Richard H. Kramer from the University of California, Berkeley.
Kramer and his Berkeley colleagues, including chemist Dirk Trauner and neurobiologist Ehud Y. Isacoff, have co-opted synthetic switches to render neurons sensitive to light. Their work is "a great mix of chemistry and biology," says Andrew Woolley of the University of Toronto, who also develops light-activated synthetic switches.
The Berkeley team's method also relies on a light-responsive double bond. Their photoswitches contain an azobenzene group, which has a nitrogen-nitrogen double bond that converts from its trans to its cis form in response to ultraviolet light. Green light causes the bond to change back to the trans form.
In their earliest work, the Berkeley team used light-activated synthetic switches tipped with a quaternary ammonium group to reversibly plug and reopen the ion-conducting pores of K+ channels, deactivating and activating the neurons at will (C&EN, Nov. 29, 2004, page 24). Later, they changed the tip of their synthetic switches from a quaternary ammonium ion to glutamate. Using the light-activated glutamate-tipped switch, they controlled a tiny fish's response to touch by selectively controlling glutamate-gated ion channels. Glutamate-gated ion channels convert chemical messages into electrical signals.
That study was a significant advance, Trauner says, because the glutamate doesn't simply act as a channel plug, as the quaternary ammonium group did. It acts as a ligand, inducing a conformational change in the ion channel that causes it to open.
Until recently, attaching the quaternary ammonium- and glutamate-based switches to neuronal ion channels required that the channels be modified to contain a cysteine attachment point. In those earlier cases, including the experiments with fish, the team used genetic engineering to express the cysteine-modified channel proteins in specific neurons.
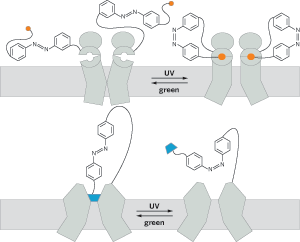
Light-activated switches can open and close different types of ion channels on demand. Ultraviolet light isomerizes a trans-azobenzene tether to its cis form (top), swinging glutamate (orange) into an ion channel's binding pocket. The isomerization induces a conformational change in the ion channel that opens it. The same isomerization (bottom) removes a quaternary ammonium plug (blue) from a K+ channel, allowing ions to pass.
THIS MONTH, however, the team reported a second-generation quaternary ammonium-containing azobenzene photoswitch that can modify naturally occurring K+ channels (Nat. Methods, DOI: 10.1038/nmeth.1187). They swapped a maleimide, the part of their original switch that attaches it to cysteine, with an acrylamide, which reacts not only with cysteine but also with other electron-rich amino acids. The switch's quaternary ammonium ion makes it capable of plugging K+ channels. But by eliminating the need for genetic engineering, there's no obvious way to target this type of switch to specific neurons. That's something the team is still working on, Trauner says.
The team injected the acrylamide-based switch into rat eyes, where it conferred light sensitivity on nerve cells in the retina that do not normally respond to light. Kramer tells C&EN that photoswitches might be useful for restoring sight in people with vision loss caused by diseases that kill specialized vision cells in the retina, but more work is needed to attain this goal.
For use in organs or parts other than the eye, however, photoswitches and light-sensitive proteins share a big limitation. The wavelengths of light currently used to flip these switches on and off don't penetrate deeply into most body tissues. That's one reason why researchers are working primarily with worms and fish with transparent bodies. "You can't get that light into very much of the brain," Weill Cornell's Ryan says. On the other hand, switches that respond to longer wavelengths, at the red end of the visible spectrum and slightly toward infrared, could be activated in living tissues because those wavelengths can penetrate living tissues to some extent. That's one strategy that research groups, including the Berkeley team, are pursuing.
Changing the wavelengths to which retinal-gated proteins respond may require screening mutations in the proteins. But researchers already know a bit about how to modify azobenzene's properties, according to Woolley. Knowledge about azobenzene chemistry and color properties is immense, dating back to the 19th-century dye industry. "Perhaps this knowledge base can be tapped to aid the design of switches with ideal properties," he says.
Regardless of how they may need to be tweaked to improve performance, retinal-gated proteins and synthetic switches are complementary, Trauner emphasizes. "There are probably some scenarios where our system is better and some where channelrhodopsin is better," he says.
Ryan agrees that the technology is still at a very early stage. "The experiments we keep seeing are proof of concept," he says. The neurons researchers have been targeting so far, he explains, have well-known roles. "What we're waiting for is the killer app," Ryan says, referring to an experiment that harnesses light-activated nerve cells to pose new questions or to answer long-standing ones.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter